Functional role of microvesicles in gastrointestinal malignancies
Introduction
Microvesicles (MVs) are small membrane enclosed bodies, which contain various molecules and are found circulating throughout the body. MVs are derived from the plasma membrane of various cell types and released by outward budding and fission of the plasma membrane (1). After fusion with the plasma membrane, MVs are shed from the cell surface of activated cells. Therefore, microvesicles may be secreted by activated malignant and normal cells and play a role in cellular communication during cancer development. Microvesicle size ranges from 200 to greater than 1,000 nm, derived directly from the plasma membrane and can take on various shapes (2). Microvesicles are sometimes mistakenly classified as exosomes. Exosomes are smaller vesicle structures which are between 50 and 80 nm and are typically uniform in shape (2). Microvesicles are formed within late endocytic components called multivesicular bodies, and are secreted upon fusion with the plasma membrane.
The distinct difference between microvesicles and exosomes can be seen during their respective formation and release. Exosomes are created intracellularly and pass through the multivesticular body before fusing with the plasma membrane and releasing their contents (3,4). Microvesicles are formed directly from the parent cell’s plasma membrane and travel throughout the body in their membrane enclosed sacs to release their contents either extracellularly or intracellularly (5). Derivation of microvesicles from a particular cell allows the microvesicles to retain similar characteristics to the cell from which it originates (2).
Microvesicles are found in many bodily fluids, including urine, ascetic fluid and peripheral blood (6-9). The contents of microvesicles range widely from critical proteins to minerals and to toxins. Because microvesicles retain some properties of their parent cells, microvesicles are important messengers between the parent cell and their targets. For example, skeletal cells release microvesicles, which initiate bone mineralization or demineralization (10).
Because of their extensive travel throughout the body and the characteristics retained by the parent cell, microvesicles are thought to contribute to tumor invasion and metastasis as well as inflammation, coagulation and stem cell renewal and expansion. In addition, some microvesicles are thought to release proteins, which damage and/or degrade the extracellular matrix in order to allow for tumor progression.
Gastrointestinal (GI) malignancies are a serious concern worldwide affecting millions of patients. GI cancer encompasses the esophagus, stomach, biliary apparatus, pancreas, bowels, and anus. MVs derived from GI cancer cells may contribute to the tumor development and progression, and may represent one of the critical components that support growth and expansion of GI malignancies. Therefore, MVs play an essential role in GI cancer as well as other cancers and targeting could help in the battle to alleviate these often-fatal diseases.
Microvesicle structure and biogenesis
Mechanisms for formation and release
Typically, microvesicles are shed when the body is in a disease state. The characteristics of a microvesicle are mostly formed during the initial budding process. Meanwhile, MVs may also be released into the extracellular milieu with consequences for the surrounding environment. For instance, microvesicles derived from neutrophils are packed with cytokines that first release anti-inflammatory molecules and can function as pro-inflammatory mediators (11,12). Therefore, understanding the formation of a microvesicle is essential to understanding their behavior.
Most of a microvesicle’s characteristics are formed during the budding process (Figure 1). The microvessicle initially buds from its parent cell and the bud is then separated from the parent cell by a fission event (2). To separate the microvesicle from the parent cell, contractile machinery within the cleavage furrow draws the opposing membranes towards one another and then pinches off the membrane connection (13). These events are similar to those associated with viral blebbing (14,15). Microvesicles can be differentiated from apoptotic blebs by the lack of cytosolic organelles or nuclear fragments.
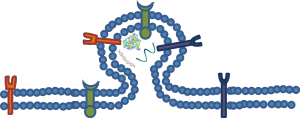
Impact of other cellular mechanisms
Membrane lipids play a key role in the mechanism of microvesicle release. For example, membrane lipids, in an attempt to stabilize membrane-bending forces, can cause the plasma membrane to release microvesicles (16). There is a very low probability that spontaneous membrane curvature is the driving force behind formation because of curvature and membrane-fusion energy constraints (17). In order to overcome these physical limitations, proteins can be utilized to alter membrane curvature through numerous mechanisms. One way that proteins accomplish this is by exerting a localized force upon the membrane to generate the curvature needed to start the budding process. In other cases, proteins can directly bind to the plasma membrane or insert amphipathic moieties into the lipid matrix (18,19). These amphipathic moieties act as a wedge which forces curvature as a result of increasing the surface area on one side of the phospholipid bilayer. Another way is that contractile proteins add or remove tensile forces to one side of the plasma membrane which creates structural asymmetry, thus causing bending (20). Proteins can also directly or indirectly regulate the composition of the lipids and the symmetry of the plasma membrane (21,22). By influencing the composition of the lipids, mainly through distribution and relocation, forces on the plasma membrane can be altered or unevenly distributed. This input from proteins, which causes structural disturbances of the plasma membrane, could also influence the ultimate size and structure of microvesicles.
Various studies have shown the microvesicle formation requires energy input, RNA synthesis and protein translation and furthermore, takes place at specific locations in the membrane, which are enriched with various lipids and proteins (20,22).
The cytoskeleton plays an important role in microvesicle formation. It has been shown that microvesicle shedding can be induced by colchicine, vinblastine and cold temperatures in P815 cells (23). This indicates that microtubule inhibition can cause microvesicle creation. This could possibly be the result of localized rupture of the plasma membrane and subsequent blebbing as a result of hydrostatic pressure differences (1). This type of blebbing is not unique to microvesicles and has been shown to be present in apoptosis, migration, and cytokinesis. Microtubules are actin positive, whereas microvesicles are only transiently associated with the actin cytoskeleton during retraction (19,24).
Contractile proteins are also important to the formation of microvesicles. They can conceivably add tensile or contractile forces to one leaflet of the membrane, creating a structural asymmetry that would lead to bending (25,26). Phosphorylated myosin light chain kinase has been shown to be present while microvesicles are being formed and myosin 1a has been shown to be necessary for microvesicle formation (20,22,27). Microvesicles appear to be contracted internally by contractile proteins, which act as a drawstring (1).
Composition and contents of microvesicles
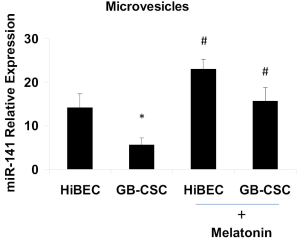
Microvesicles are composed of a plasma membrane and proteins, nucleic acids, cytokines, oncogene and growth factor receptors, integrin receptors, proteases and/or major histocompatibility complex (MHC) class I molecules. The plasma membrane derived from the parent cell incorporates some but not all plasma membrane proteins and those that are incorporated conserve the topology of the parent cell’s plasma membrane. Studies have suggested that an array of proteins is selectively incorporated into microvesicles including proteins that are transported via the ARK6-regulated endosome-recycling pathway (20,28). Conceivably, specialized endosomes are involved in selectively targeting cargo to move into a larger microvesicles in tumor cells. Microvesicles have been found to contain nucleic acids, and in particular, miRNAs (21,29,30). We have demonstrated that miR-141 is silenced in human gallbladder cancer stem cell derived microvesicles, and its expression in human cholangiocytes derived MVs is regulated by melatonin (Figure 2). Some studies suggest that the site of microvesicle shedding could be a convergence point for membrane trafficking pathways, which are directing specialized cargo to the microvesicles (1).
Microvesicles in cancer progression
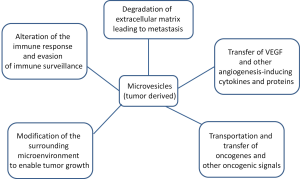
In order for tumors to proliferate, tumor cells must break down the surrounding extracellular matrix to allow for angiogenesis and metastasis. Microvesicles are an important component of tumor proliferation (Figure 3). The enhanced release of microvesicles from tumor cells has been shown to correlate increased growth and invasiveness of the tumor both in vitro and in vivo.
Evasion of immune surveillance
The immune system is very adept at finding and eliminating abnormal material in the body. Unfortunately, tumor cells have the ability to evade the immune system, which will allow their proliferation. This is accomplished through mechanisms which silence the tumor cells immunogenic profile and activate immunosuppressive pathways. Experiments with microvesicles produced by melanoma or colorectal cancer cells showed that when directly fused with monocytes, inhibited the differentiation of the monocytes into antigen presenting cells and the monocytes instead secrete immunosuppressive cytokines (31). Tumor cells have also been known to release microvesicles containing CD95 ligand, which induces, activates anti-tumor T-cell apoptosis (31,32). Tumor cells can theoretically fuse with microvesicles from normal cells in order to camouflage themselves by incorporating lipids and membrane-specific proteins of normal cells (1).
Tumor microenvironment
Tumor cells actively modify their surrounding environment to promote their own growth and proliferation (6). The ability to degrade the extracellular matrix is a vital component of a tumor’s ability to promote angiogenesis and metastasis. It is thought by many that there is a direct correlation between invasive microvesicles and the proliferation of tumor cells. Many of the microvesicles shed by tumor cells contain proteases, which help degrade the extracellular matrix. Microvesicles shed by tumor cells have been found to contain MMP2, MMP9, MT1-MMP and the proenzymes urokinase-type plasminogen activator (uPA) and extracellular matrix metalloproteinase inducer (EMMPRIN) (6,33-36). These molecules are known to degrade the extracellular matrix in various ways. uPA individually catalyzes the transformation of plasminogen into plasmin, which initiates the conversion of matrix metalloproteinase (MMP) proenzymes into their active form. The active enzymes degrade fibrin within the ECM. The weakening of the ECM and its subsequent decrease in function allows for the increase of cellular tumor proliferation to surrounding tissues (34,35).
Angiogenesis
In order for tumors to grow larger than a small size, a blood supply must be created to deliver nutrients to the cells and remove wastes. The tumor accomplishes this by angiogenesis, the growth of new blood vessels from pre-existing blood vessels (36,37). Several studies describe the shedding of microvesicles containing pro-angiogenic factors by tumor cells. Vascular endothelial growth factor (VEGF) as well as various MMPs have been found in tumor-derived microvesicles (38). Although growth factors and proteases are commonly found, microRNAs (miRNAs) have been found in microvesicles as well. miRNAs from these microvesicles modify the translational profile of endothelial cells which stimulates tubule formation and promotes acquisition of an angiogenic phenotype (39). Microvesicles derived from tumor cells have also been shown to trigger endothelial cells to release their own microvesicles containing VEGF and sphingomylein which promote angiogenesis (40).
Microvesicles and tumor-triggered blood coagulation
Mortality related to cancer can often times be associated with a wide variety of hematological complications categorized as thromboembolism (41). Tissue factor (TF) is the molecule associated most closely with hypercoagulation in cancer patients. TF works by coupling with factors VII and/or VIIIa, which activates thrombin and leads to deposition of fibrin. Most microvesicles containing TF have been shown to be derived from tumor cells and these microvesicles have been shown to increase the risk of thromboembolic disease in malignancy (42).
Microvesicles in gastrointesional cancer
Gastrointestinal cancer is a leading cause of death around the world. It is comprised of cancer of the gastrointestinal system, which includes the esophagus, stomach, small intestine, colon, liver, biliary system, pancreas, bowels, and anus. The National Cancer Institute estimates that approximately 25% of all cancer is of gastrointestinal origin, with the majority originating in the colon and/or rectum. Malignancy can occur anywhere in the gastrointestinal system and affect any one of the gastrointestinal organs.
Studies have shown that patients with gastrointestinal cancer had a larger number of microvesicles compared to patients with other gastrointestinal diseases (43). Proliferation of microvesicles has been shown to exist in several different kinds of gastrointestinal cancer. The esophagus has exhibited microvesicle activity in the simple junctions both in the striated muscle fibers and the smooth layers (44). Also, esophageal cells have also been induced to secrete microvesicles upon incubation with gastric bile or duodenal juice (45).
Microvesicles have also been seen in patients with gastric cancer. The microvesicles present in patients with gastric cancer have been shown to have an increased membrane expression of CCR6 which downregulates T-Cell activation and HER-2/neu, which activates proliferation and downregulates apoptosis (46). Gastrointestinal stromal tumors have been shown to secrete microvesicles containing Fas ligand (CD95L), which is known to promote cell death (32,47).
Microvesicles can be beneficial as well. In a recent study, in vivo administration of microvesicles derived from human adult liver stem cells directly into tumors showed regression of ectopic tumors in severe combined immunodeficiency (SCID) mice. The mechanism of regression is thought to be mediated by the delivery of miRNAs to the tumor cells (48). miRNAs and other non-coding RNAs are critical mediators for functional MVs to promote specific tumor growth. Colorectal cells are also able to secrete microvesicles that are enriched in cell cycle miRNAs, which can cause increased proliferation of tumors and induce the surrounding tissue to form tumors as well (49). Colorectal tumor cells escape detection from the immune system through microvesicle secretion. These microvesicles contain tumor necrosis-related apoptosis - inducing ligand as well as Fas ligand, which induce T-cell apoptosis (50). Microvesicles derived from colorectal cancer carcinoma cells promote the differentiation of monocytes to myeloid-derived suppressor cells. This differentiation supports the growth of colorectal tumors and evasion from the immune system (31).
Conclusions
Microvesicles are the important aspect of intercellular signaling in cell and cancer biology. Various types of cells communicate with each another via microvesicles. These microvesicles contain proteins, nucleic acids and cytokines as communication while maintaining the membrane and proteins from the parent cell, which is most likely used to identify the microvesicle when it gets to its target. While they have been shown in normal cells, it is important to note that tumor cells are the largest producer of microvesicles. Tumor cells use microvesicles to not only communicate with other cells, but to camouflage themselves from the immune system. Because microvesicles seem to be an important part of carcinogenesis, they could become an important target for therapeutic approach to GI malignancies.
Future prospectives
Microvesicles could become a major therapeutic target for gastrointestinal and other types of tumors. One way in which microvesicles could be targeted is to halt their shedding. If this is accomplished, critical components of tumor growth and proliferation would be halted such as angiogenesis and degradation of the extracellular matrix. Communication between the tumor and surrounding tissues and cells would be halted as well. These things would not allow the tumor to develop further and would keep the tumor’s growth to a minimum. Microvesicles could also be targeted by targeting the plasma membrane of tumor cells. If a foreign body marker was inserted into the plasma membrane of tumor cells, this would more than likely be incorporated into the microvesicles, which would allow the immune system to target them. Another possibility is to target proteins responsible for altering plasma membrane curvature. Without these proteins, the membrane would be unable to exert the local pressures required to cause microvesicle formation.
Acknowledgements
Portion of this review article was supported by the NIH R01 grant DK062975 to G. Alpini, VA Merit review to F. Meng and Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Muralidharan-Chari V, Clancy JW, Sedgwick A, et al. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010;123:1603-11. [PubMed]
- Muralidharan-Chari V, Clancy J, Plou C, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 2009;19:1875-85. [PubMed]
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic 2008;9:871-81. [PubMed]
- Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009;21:575-81. [PubMed]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol 2009;19:43-51. [PubMed]
- Graves LE, Ariztia EV, Navari JR, et al. Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 2004;64:7045-9. [PubMed]
- Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev 2007;21:157-71. [PubMed]
- Smalley DM, Sheman NE, Nelson K, et al. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J Proteome Res 2008;7:2088-96. [PubMed]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008;110:13-21. [PubMed]
- Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci 2005;10:822-37. [PubMed]
- Köppler B, Cohen C, Schlöndorff D, et al. Differential mechanisms of microparticle transfer toB cells and monocytes: anti-inflammatory propertiesof microparticles. Eur J Immunol 2006;36:648-60. [PubMed]
- Mack M, Kleinschmidt A, Brühl H, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 2000;6:769-75. [PubMed]
- Schweitzer JK, D’Souza-Schorey C. Finishing the job: cytoskeletal and membrane events bring cytokinesis to an end. Exp Cell Res 2004;295:1-8. [PubMed]
- Chazal N, Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev 2003;67:226-37. [PubMed]
- Morita E, Sundquist WI. Retrovirus budding. Annu Rev Cell Dev Biol 2004;20:395-425. [PubMed]
- Corbeil D, Röper K, Fargeas CA, et al. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic 2001;2:82-91. [PubMed]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature 2005;438:932-6. [PubMed]
- Charras GT, Yarrow JC, Horton MA, et al. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 2005;435:365-9. [PubMed]
- Del Conde I, Shrimpton CN, Thiagarajan P, et al. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005;106:1604-11. [PubMed]
- Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem 2003;278:41573-6. [PubMed]
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, et al. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother 2006;55:808-18. [PubMed]
- Farsad K, De Camilli P. Mechanisms of membrane deformation. Curr Opin Cell Biol 2003;15:372-81. [PubMed]
- Liepins A. Possible role of microtubules in tumor cell surface membrane shedding, permeability, and lympholysis. Cell Immunol 1983;76:120-8. [PubMed]
- Hugel B, Martínez MC, Kunzelmann C, et al. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22-7. [PubMed]
- Huttner WB, Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr Opin Cell Biol 2001;13:478-84. [PubMed]
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol 2006;7:9-19. [PubMed]
- Bausch AR, Ziemann F, Boulbitch AA, et al. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys J 1998;75:2038-49. [PubMed]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 2006;7:347-58. [PubMed]
- Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470-6. [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [PubMed]
- Valenti R, Huber V, Iero M, et al. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res 2007;67:2912-5. [PubMed]
- Andreola G, Rivoltini L, Castelli C, et al. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med 2002;195:1303-16. [PubMed]
- Hotary K, Li XY, Allen E, et al. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev 2006;20:2673-86. [PubMed]
- Ginestra A, Miceli D, Dolo V, et al. Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res 1999;19:3439-45. [PubMed]
- Ginestra A, La Placa MD, Saladino F, et al. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res 1998;18:3433-7. [PubMed]
- Rauch U, Antoniak S. Tissue factor-positive microparticles in blood associated with coagulopathy in cancer. Thromb Haemost 2007;97:9-10. [PubMed]
- Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost 2007;97:119-23. [PubMed]
- Taraboletti G, D’Ascenzo S, Giusti I, et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia 2006;8:96-103. [PubMed]
- Hunter MP, Ismail N, Zhang X, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One 2008;3:e3694. [PubMed]
- Kim CW, Lee HM, Lee TH, et al. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res 2002;62:6312-7. [PubMed]
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol 2007;62:126-36. [PubMed]
- Giesen PL, Rauch U, Bohrmann B, et al. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A 1999;96:2311-5. [PubMed]
- Jansa R, Sustar V, Frank M, et al. Number of microvesicles in peripheral blood and ability of plasma to induce adhesion between phospholipid membranes in 19 patients with gastrointestinal diseases. Blood Cells Mol Dis 2008;41:124-32. [PubMed]
- Bazhenov DV. Intercellular relations in esophageal muscle tissues. Arkh Anat Gistol Embriol 1987;93:77-82. [PubMed]
- Bateson MC, Hopwood D, Milne G, et al. Oesophageal epithelial ultrastructure after incubation with gastrointestinal fluids and their components. J Pathol 1981;133:33-51. [PubMed]
- Baran J, Baj-Krzyworzeka M, Weglarczyk K, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother 2010;59:841-50. [PubMed]
- Rikhof B, van der Graaf WT, Meijer C, et al. Abundant Fas expression by gastrointestinal stromal tumours may serve as a therapeutic target for MegaFasL. Br J Cancer 2008;99:1600-6. [PubMed]
- Fonsato V, Collino F, Herrera MB, et al. Human Liver Stem Cell-Derived Microvesicles Inhibit Hepatoma Growth in SCID Mice by Delivering Antitumor MicroRNAs. Stem Cells 2012;30:1985-98. [PubMed]
- Hong SN, Kim JH, Choe WH, et al. Circulating vitamin D and colorectal adenoma in asymptomatic average-risk individuals who underwent first screening colonoscopy: a case-control study. Dig Dis Sci 2012;57:753-63. [PubMed]
- Huber V, Fais S, Iero M, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology 2005;128:1796-804. [PubMed]


