Toxicity management of immunotherapy for patients with metastatic melanoma
Introduction
In recent years the development of immune checkpoint inhibitors has restored the ‘faith’ in immunotherapy as a central therapeutic strategy. These agents dramatically improved the outcome of metastatic melanoma patients (1). The modulation of the immune response in order to harness antineoplastic activity has long been investigated in the treatment of melanoma with more pitfalls than successes. The sole representative of activity is the response with high-dose interleukin-2 in a small subset of metastatic melanoma patients, achieved at the expense of significant toxicity (2). A new therapeutic avenue has been opened with the discovery of immune checkpoints responsible for the regulation of the immune response and has led to the development of a new class of agents, the immune checkpoint inhibitors. These are immunomodulatory monoclonal antibodies that act via the blockade of specific immune response receptors, thus enhancing the immune system. The main targets are the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) receptor on T lymphocytes and the PD-1 receptor (programmed cell death-1), as well as the PD-1 ligand (PD-L1).
The first checkpoint inhibitor was ipilimumab, an antibody against CTLA-4. Ipilimumab, extensively investigated in phase III studies against standard chemotherapy, is now approved as front-line therapy of patients with metastatic melanoma, based on significant improvements in overall survival (3,4). Another anti-CTLA-4 antibody, tremelimumab, did not show overall survival benefit (5). The clinical development of Ipilimumab was long, including a large number of patients, and thus allowing not only for a clear understanding of the specific toxicities observed with this agent but also for the development of comprehensive guidelines for toxicity management.
The second class of inhibitors are directed against PD-1 and its ligand (PD-L1) and several antibodies have now been developed and are investigated not only in melanoma but also in many other solid tumors [non-small cell lung cancer (NSCLC), renal and breast cancer, etc.] (6,7). The more advanced in clinical development inhibitors include nivolumab and pembrolizumab, both against PD-1, which have been extensively studied against standard chemotherapy but also compared with ipilimumab and have proven to offer superior responses and favorable toxicity profiles (8-11). Both are already approved for melanoma, while nivolumab is also approved for 2nd line treatment of squamous and non-squamous NSCLC (12), whereas pembrolizumab is approved in the same indication in tumors that express PD-L1. Pembrolizumab is approved for use with a companion diagnostic, the PD-L1 IHC 22C3 pharmDx test, the first test designed to detect PD-L1 expression in non-small cell lung tumors (13). Atezolizumab is an agent targeting PD-L1, which is also under clinical development for NSCLC but also have shown promising activity in other cancers, and recently approved in urothelial cancer (14).
The unique side effects of checkpoint inhibitors are uniformly termed as immune-related adverse events (irAEs). These include a range of mainly dermatologic, gastrointestinal (GI), endocrine and hepatic toxicities, as well as several other less common inflammatory events. All these adverse events have variable times of onset, they have an autoimmune etiology and they need careful monitoring, follow-up and management. With appropriate and timely treatment, these toxicities are usually reversible, but they can become severe and even life-threatening if they are not recognized early enough. These checkpoint inhibitor side effects are reviewed here and their management is presented based on clinical experience and published guidelines, algorithms and recommendations, which refer mainly to the knowledge obtained from the use of ipilimumab (15).
General considerations for the management of common irAEs
Among patients receiving ipilimumab, the most commonly reported adverse events included diarrhea, colitis, fatigue, pruritus, rash and endocrinopathies (16). For patients treated with PD-1 inhibitors, common adverse events include fatigue, rash, diarrhea, pruritus, arthralgia and constipation (17-19). Timely communication between patients, caregivers and physicians is essential for the early recognition and management of irAEs. There have been several treatment algorithms developed for ipilimumab toxicities and most of them can be applied for the toxicities observed with anti-PD-1 agents, as well (20,21). With the broader use of anti-PD-1 agents, it is important to be able to evaluate how prior ipilimumab exposure and toxicity may impact the safe use of anti-PD-1 agents. So far it seems that previous treatment with Ipilimumab does not negatively affect the use of nivolumab or pembrolizumab, however, less is known about the impact of previous severe toxicities with ipilimumab (9,22).
General principles for the optimal management of irAEs include the early recognition and the appropriately-timed use of immunosuppressive agents, such as steroids or anti-TNF-α, based on the severity of the event. IrAEs of any grade with ipilimumab occur in the majority of patients, as seen in 64.2% of patients in a pooled analysis of 14 phase I–III studies of ipilimumab (16). Most toxicities are mild to moderate (grade 1–2), involve mainly skin and GI events, while treatment-related deaths are very rarely seen (<1% of patients). Furthermore, the incidence and severity of ipilimumab toxicities appear to be dose related (23).
Onset and resolution of irAEs with ipilimumab
The onset and outcome of irAEs with ipilimumab seem to vary according to the organs involved and although most occur within the first 3 months of treatment, there are some specific toxicities reported months after the end of ipilimumab therapy. The majority of irAEs of any grade (86%), observed in the phase III study of ipilimumab vs. gp100, were recorded within the first 3 months of therapy and the majority also resolved within 3 months (24,25). From the experience so far from thousands of patients treated in ipilimumab studies, it is evident that dermatologic irAEs appear usually after 2–3 weeks and typically resolve fast, GI and hepatic irAEs appear after 6–7 weeks, while endocrinopathies can be diagnosed even after 9 weeks and can take a long time to resolve or in some cases might even be irreversible, like it is the case for most hypophysitis observed (15,25).
Specific toxicities
Dermatologic adverse events
Skin toxicity is observed in almost half of the patients treated with ipilimumab (44%) and in the vast majority it is of grade 1–2. Severe skin toxicity (grade 3–4) is recorded in less than 2% of the cases.
Rash and pruritus
Rash is usually maculo-papular and appears between 3–6 weeks of ipilimumab treatment. It has been reported in approximately 20% of the patients in most studies. Most management algorithms suggest that with the appearance of any generalized rash, physicians should exclude any non-immune related cause, discontinue and avoid any concomitant medications that could cause skin reactions, such as antibiotics, anticonvulsants, or proton pump inhibitors and assess the severity (grading) of the rash according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) criteria. In most cases the rash is grade 1 (involves <10% of the body surface area) and in such cases increased monitoring and symptomatic treatment until resolution are usually sufficient. In general no change in the ipilimumab treatment schedule is recommended, although skipping the next ipilimumab dose until the rash is completely resolved, based on clinical judgement, could be considered. Topical and/or oral steroid therapy should be offered for persistent or recurring grade 2 rash (with or without pruritus). Topical treatment could include betamethasone 0.1% or clobetasol 0.05%. Pruritus might appear alone or accompany low grade skin events in about a quarter of patients receiving ipilimumab and usually responds to antihistamines per os.
For more severe events, grade 3–4 (symptomatic lesions with involvement of >30% of body surface area, it is strongly recommended to use high-dose steroid therapy intravenously (methylprednisolone 1–2 mg/kg/day, or similar) followed by oral steroids on improvement, which should be gradually tapered over 4 weeks. Such events are rare, and a specialist consult is recommended, while further ipilimumab dosing should be withheld until resolution, although such severe events might lead to permanent ipilimumab discontinuation. If symptoms do not respond within 5–7 days of steroid intervention, then an alternative immunosuppression therapy should be considered, such as tacrolimus, infliximab (26,27).
Vitiligo
Vitiligo has been observed as a response to several treatments in patients with melanoma and it is usually thought to be a ‘marker’ of response to treatment or improved outcome (28). In studies with ipilimumab, vitiligo has also been observed but it is not clear if its occurrence is related to benefit from the ipilimumab treatment.
GI adverse events
Diarrhea and colitis
One of the most discussed irAEs of ipilimumab is diarrhea. Different rates of diarrhea have been reported according to the dose of ipilimumab used in trials and it seems that higher doses are associated with higher rates of diarrhea. Most studies report at least 30% of diarrheic events, which commonly present after 5 weeks of treatment and in most cases are grade 1. Diarrhea results from infiltration of the intestinal mucosa by immune cells following immune activation by the checkpoint inhibitor treatment. Colitis is the severe consequence of diarrhea and there have been reports of bowel perforation and deaths due to colitis.
There are several published algorithms for the management of diarrhea, as the one included in the Summary of Product Characteristics (SPC) of ipilimumab. Most guidelines include recommendations for management based on grading and severity and sequential algorithms. Since 2005, specific guidelines for diarrhea management have been implemented in all the clinical studies involving ipilimumab and soon it was realized that this intervention reduced the incidence of severe GI toxicities and perforations even when higher doses of ipilimumab were used (29,30). This detailed guidance algorithm can be found in the SPC of ipilimumab (20). In general, when a patient on ipilimumab therapy presents with diarrhea or blood in stools, initially we have to rule out non-immune related causes, such as microbial infections. In such cases specific treatment is administered and when the event is resolved ipilimumab can be continued. The grade of the event should be properly assessed. In grade 1 diarrhea it is recommended to treat symptomatically without steroids, administer loperamide 2 mg per os q 4–6 hours, anti-diarrheic diet and hydration and monitor closely until resolution.
In grade 2 diarrhea, that is increase to 4–6 bowel movements, or abdominal pain or blood in stools, if initial symptomatic treatment without steroids is not effective, stool white blood cell (WBC) should be send and stool calprotectin and endoscopy should be considered. Treatment with oral budesonide or other moderate dose steroid should be initiated. Steroid tapering should be gradual and definitely not shorter than 30 days, since premature stopping might lead to relapse. In grade 3 colitis (increase of ≥7 stools per day over baseline, incontinence, need for hospitalization for IV fluids for ≥24 h) then treatment with high dose steroids is required (methylprednisolone 1–2 mg/kg/day IV until improvement with a slow tapering for at least a month). If no response is seen in 1 week, then it is recommended to consider immunosuppressive therapy with anti-TNF inhibitors (5 mg/kg remicade, infliximab), which are approved for the treatment of colitis (29,30).
Hepatotoxicity
Liver toxicity has been reported in about 5% of the patients, usually appears after 6 weeks of treatment and consists of liver enzymes and bilirubin elevations or even acute hepatitis. Close monitoring of liver function tests (LFTs) is essential and if LFTs or bilirubin are elevated >2 times of normal baseline, then monitoring should be intensified and work-up for autoimmunity should be initiated. This should include careful clinical examination, blood tests for antinuclear antibody (ANA), anti-smooth muscle antibody (SMA), LFTs, total bilirubin and creatinine, which should be repeated every 2–3 days. Also in order to rule out other causes for hepatitis, one should consider radiological imaging or even a liver biopsy. If LFTs continue rising >8× upper limit normal (ULN) or immune-mediated hepatitis is clinically possible, therapeutic interventions with a steroid treatment are recommended (16,31). The following algorithm is proposed:
- Admit patient to hospital for evaluation and close monitoring;
- Stop further ipilimumab until hepatotoxicity is resolved. Consider permanent discontinuation of ipilimumab;
- Start at least 120 mg methylprednisolone per day, IV, as a single or divided dose;
- Check LFTs and bilirubin daily until stable or showing signs of improvement for at least 3 consecutive days;
- If no decrease in LFTs after 3 days or rebound hepatitis occurs, despite treatment with corticosteroids, then add an immunosuppressive agent;
- If no improvement after 5 to 7 days, consider adding 0.10–0.15 mg/kg/day tacrolimus (trough level 5–20 ng/mL);
- If target trough level is achieved with tacrolimus but no improvement is observed after 5–7 days, consider infliximab, 5 mg/kg, as a single dose;
- Continue to check LFTs daily for at least 2 weeks to monitor sustai/ned response to treatment.
Endocrinopathies
The fairly common advent of endocrine disorders in patients receiving ipilimumab has led to recommendations for routine monitoring of thyroid function at least during ipilimumab treatment and close monitoring of other endocrine function tests. Clinical signs and symptoms that could make the clinician suspect an underlying endocrinopathy include, among others, fatigue, weakness, anorexia, headache, visual field defects, nausea, fever, lethargy, impotence, amenorrhea, new onset atrial fibrillation, hypotension, hypoglycemia or hyponatremia. Thyroid disorders can be detected with the patient being asymptomatic, from elevated TSH or low T3, T4 levels. These are usually easily corrected with hormone replacement.
Hypophysitis is one of the irAEs that can remain undetected since the symptoms might be vague, such as fatigue, hypotension or myalgias, and only if the clinician is aware of the risk, laboratory tests might be considered and thus diagnose this irAE. Management includes hormone replacement, according to hormone dysfunction (thyroxine, testosterone, estradiol, or more commonly steroids, such as hydrocortisone). Endocrinopathies are generally managed with a short course of high dose steroid treatment to reverse inflammation, appropriate hormone replacement to reverse endocrinopathy, while an endocrinologist should be involved and consulted as soon as an endocrinopathy is suspected. Immune-related endocrinopathies are usually detected after at least 6 weeks of treatment and they may take months to resolve or even be irreversible (32-34).
Immune-related neuropathies
Autoimmune neuropathies are rare (<1%) but could range from mild paresthesias to severe neurologic syndromes (35-37). When signs of either sensory or motor neuropathy are present, the diagnostic algorithm should initially rule out other causes, such as infection, metabolic abnormalities or other drugs. A neurologist should be consulted and tests, such as an electromyogram and nerve conduction studies should be performed in order to fully characterize the neuropathy. Symptoms should be treated accordingly and ipilimumab skipped until resolution for low grade events. If neuropathy is considered to be more than grade 2, ipilimumab should be stopped and treatment with oral or iv steroids or even other immunosuppressive agents should be initiated based on symptom severity.
Ocular toxicity
Eye toxicity is also rare (<1%) and it includes conjunctivitis or uveitis, which usually respond well to topical steroid treatment. It goes without saying that an ophthalmologist should be consulted (38).
Management of less frequent irAEs
Other less common toxicities have been attributed to ipilimumab treatment and although rare, physicians should be aware of the risk of their occurrence. In general, their management follows the same guidelines of prompt symptom control and timely steroid use or immunosuppression.
Pneumonitis
Any new episodes of cough or dyspnea that were not pre-existing, in a patient undergoing immunotherapy, should be suspected for pneumonitis. This is an irAE that can occur both with ipilimumab or anti-PD-1 agents (39). Because the onset and symptoms of pneumonitis are often vague and diagnosis is often delayed, clinicians should be aware of this and consider diagnostic radiology (X-rays, CT scans) or even bronchoscopy to rule out other causes. Severe pneumonitis is very rare with ipilimumab but it has been reported with anti-PD-1 agents (1%), especially in lung cancer patients, where even deaths related to immune-onset pneumonitis have been reported.
In general, pneumonitis management involves prompt high-dose steroid initiation and close monitoring of symptoms, oxygen needs and radiological findings for appropriate slow tapering or rarely further immunosuppressive interventions.
Renal toxicity
Autoimmune nephritis and renal failure have been reported with ipilimumab but also with anti-PD-1 agents (40). Management involves prompt recognition and initiation of high-dose steroids. Renal dysfunctions or just increases in creatinine are more often reported with nivolumab (1–22% in some studies). Specific algorithms for the management of renal toxicity from ipilimumab and anti-PD-1 agents have been developed, which include close monitoring of creatinine, steroid administration and immunotherapy interruption until resolution. Severe toxicity might require high-dose steroids intravenously and definitely permanent discontinuation of the immunotherapy agent.
Other rare irAEs
Asymptomatic elevation of the enzymes amylase and lipase has also been reported with both ipilimumab and anti-PD-1 agents. The diagnosis of non-pre-existing diabetes should lead to endocrinology consultation and treatment as required.
Some other rare irAEs have been reported, such as blood cell-penias or blood cell aplasias (i.e., thrombocytopenia), Guillain-Barre syndrome, encephalopathies or transverse myelitis (41).
Further considerations on the management of irAEs
- There is some evidence suggesting that patients suffering toxicity are deriving most efficacy from immune checkpoint inhibitor therapies, however, this is not generally accepted and needs to be prospectively validated;
- It is well established that steroids have immunosuppressive activity, and they should not be used during immunotherapy; however, this is by no means a reason not to use them in irAEs. Steroids must be promptly and timely initiated in case of irAEs, as they can save lives.
Toxicities of anti-PD-1 agents and management
As mentioned before, most guidelines and algorithms have been developed based on the large amount of clinical data from ipilimumab trials. Similar data are more limited with anti-PD-1 agents, therefore, management algorithms for these agents follow the guidance developed from ipilimumab.
Most commonly observed toxicities in studies with anti-PD-1 agents include diarrhea, although colitis, endocrinopathies, skin toxicity, fatigue and flu-like symptoms (fever, myalgias, etc.) have been observed in rare instances (6,42).
Pneumonitis and cough are reported more commonly with anti-PD-1 agents (nivolumab, pembrolizumab) than with ipilimumab, but are mostly grade 1 or 2 (43). Management should be similar to the recommendations for ipilimumab irAEs.
More specific information is now available on the AEs observed in >2,000 patients participating in completed and ongoing studies with anti-PD-1 agents; it is clear that these are caused by inflammatory mechanisms and require patient education and frequent monitoring, while they are generally manageable with timely interventions, such as steroids and/or other immunosuppressants and endocrine replacement therapy when endocrinopathies are observed. Also, several specific algorithms are being developed for the management of the anti-PD-1 AEs.
Skin toxicity
Usually the skin toxicities observed with anti-PD-1 agents include rash (14%), which is typically focal with a maculopapular appearance occurring on the trunk, back, or extremities and pruritus (10%). All observed cases have been of low or moderate grade and are successfully managed with topical steroids and anti-histamines for pruritus.
Pneumonitis
Pneumonitis is not common, it has been reported in up to 3% of the patients (all grades), but only 1% was of grade 3–4. There is no apparent relationship to tumor type treated, as pneumonitis cases have been observed in studies with multiple tumor types, including melanoma and lung and kidney cancers, although it has been suggested that pneumonitis presents more often when anti-PD-1 agents are used in patients with lung cancer than melanoma (39,43,44). Pneumonitis symptoms include cough, shortness of breath, dyspnea and fever and often involve only asymptomatic radiographic changes. A lung specialist consultation could be helpful and chest X-rays and CT scans of the thorax are necessary for diagnosis. The anti-PD-1 dose should be delayed and corticosteroids should be initiated. If symptoms are not improving within 48 h or worsening, then immunosuppressants should be added. Patients with grade 3–4 pneumonitis should be permanently discontinued from anti-PD-1 therapy. In a preliminary analysis across multiple nivolumab monotherapy studies, 12 subjects with grade 1 or 2 pneumonitis were re-treated, with 2 out of 12 subjects developing recurrent pneumonitis (17%).
Management guidance of immune-related pneumonitis is provided in Table 1.
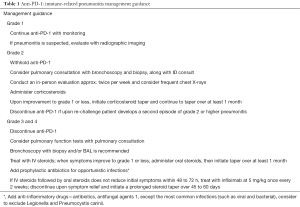
Full table
GI toxicity
Most cases of diarrhea from anti-PD-1 studies were low grade, while colitis occurred less frequently than diarrhea and there have been no GI perforations reported (45,46). Low grade diarrhea is managed symptomatically ± dose delay, while high grade cases of diarrhea/colitis have been managed with corticosteroids. It is advised that when steroids are initiated, tapering should be slow. All high grade cases reported in studies have resolved.
Hepatotoxicity
Hepatitis related to anti-PD-1 therapy is rare (47). Median time of onset reported as 89 days (range, 13–140 days). Long steroid taper is indicated, even if improvement occurs rapidly. There have been no fatal cases reported, while most cases were managed with corticosteroids with event resolution.
Endocrinopathies
Endocrinopathies reported with anti-PD-1 agents usually include hypothyroidism or hyperthyroidism, hypophysitis, adrenal insufficiency and secondary adrenocortical insufficiency, while more than one endocrine organ may be involved (47). Incidence is approximately 6% for all grades, with grade 3/4 incidence being 1%. Endocrinopathy may appear within weeks or may occur many months after treatment initiation. It is typically identified through routine periodic monitoring or as part of a work-up for associated symptoms. These symptoms are often non-specific, such as headaches, fatigue, weakness, memory loss, impotence, personality changes and visual-field impairment and their persistence should be suspicious for the occurrence of an endocrinopathy. Anti-PD-1 therapy may be continued once appropriate hormone replacement is initiated, while patients with endocrinopathy may require replacement dose steroids rather than high-dose steroids. Management guidance of immune-related thyroiditis is provided in Table 2.
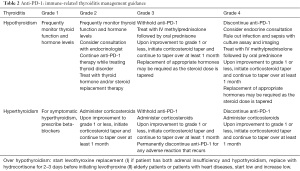
Full table
Fatigue
The most common treatment-related adverse events of any grade reported in the pivotal trials of nivolumab and pembrolizumab is fatigue, encountered in 20% of the patients. For grade 1 to 3 fatigue, non-pharmacological interventions include: energy conservation; referral for physical therapy for patients with comorbidities, recent major surgery, specific functional or anatomical deficits and substantial deconditioning; psychosocial interventions; nutritional consultation; and sleep therapy. Naps should be limited to less than 1 hour with distractions included (games, music, reading, socializing, etc.). Labor-saving techniques to not exhaust energy. Moderate level of physical activity is encouraged. For pharmacological interventions, psychostimulants (methylphenidate or modafinil) can be considered after ruling out other causes. Treating pain, emotional distress and anemia is indicated. Treatment should be optimized for sleep disfunction, nutritional deficiency and comorbidities. If grade IV toxicity occurs, treatment with anti-PD-1 should be discontinued (47).
Renal toxicity
Acute renal failure has been reported in <1% of the patients treated with nivolumab monotherapy or in combination studies for melanoma or NSCLC. Median time of onset has been reported as 43 days (range, 6–505 days) and most commonly present with elevations in serum creatinine. Steroids generally lead to clinical improvement and resolution (47). Management guidance of immune-related renal toxicity is provided in Table 3.
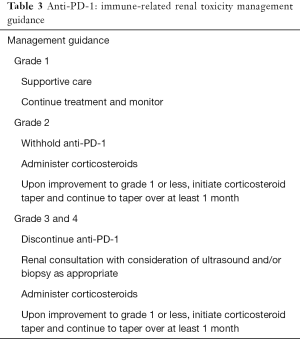
Full table
PD-L1 inhibitors
PD-L1 inhibitors are associated with very few reports of severe toxicities and are considered to be the least toxic immunotherapy agents to date. Most common irAEs observed with these agents include hyperglycemia, transaminase elevations and endocrinopathies (adrenal failure) (48). Recommendations for toxicity management follow the ones existing for ipilimumab.
Toxicities of checkpoint inhibitor combinations
Immune checkpoint inhibitors are being combined with each other or with other effective agents in an attempt to improve efficacy and long-term outcomes. Ipilimumab + nivolumab in combination have been studied in the checkmate 067 trial, which provided significant efficacy benefits but severe toxicity. The rate of grade 3–4 toxicities was 55% with the combination compared with 16% and 27% with nivolumab and ipilimumab monotherapy, respectively. All toxicities observed with the combination were similar to the ones previously described for each agent alone and were managed in a similar way (49).
Immunologic biomarkers related to immune checkpoint inhibitor toxicity
Several studies have proposed biomarkers to predict side effects of immune checkpoint inhibitor therapy, such as eosinophilia, IL-17 or gene profiling. Results are inconclusive and numbers are small, however this is a very interesting field of ongoing research (50,51).
Conclusions
Immune checkpoint inhibitors have revolutionized treatment for metastatic melanoma and are having a significant impact on many solid malignancies. Ipilimumab was the first to offer a significant survival advantage and then the anti-PD-1 agents added responses to a very encouraging potential for long-term outcome benefits. The cost of severe toxicity, initially seen with ipilimumab, is now somehow balanced and well-managed, as specific guidelines have been developed and are being broadly implemented. Furthermore, the toxicity of anti-PD-1 agents is less and the previous knowledge from ipilimumab assists in its timelier and more efficient management. Education of patients, caregivers and doctors and knowledge of toxicity management algorithms are essential for the early and prompt recognition of symptoms and the effective management of side effects. This is of particular importance, as these agents are moving fast in the treatment of earlier stages and in the adjuvant treatment of melanoma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med 2016;14:20. [Crossref] [PubMed]
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in advanced squamous cell non-small cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691-7. [Crossref] [PubMed]
- Ibrahim RA, Berman DB, de Pril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol 2011;29:abstr 8583.
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4:560-75. [PubMed]
- Keytruda® [package insert]. Whitehouse Station, NJ, USA: Merck & Co., Inc., 2015. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo
- Opdivo® [package insert]. Princeton, NJ, USA: Bristol-Myers Squibb Company, 2015. Available online: http://dailymed.nlm.nih.gov/dailymed/drugInfo
- YERVOY™ (ipilimumab) US Prescribing Information: Risk Evaluation and Mitigation Strategy (REMS). Princeton, NJ, USA: Bristol-Myers Squibb Company, 2011. Available online: http://www.hcp.yervoy.com/
- Fecher LA, Agarwala SS, Hodi FS, et al. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 2013;18:733-43. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Wolchok J, Neyns B, Linette B, et al. Ipilimumab monotherapy in patients with previously treated, advanced melanoma: a randomized, double-blind, multicenter, phase 2, dose-ranging study. Lancet Oncol 2010;11:155-64. [Crossref] [PubMed]
- Dummer R, Maio M, Hamid O, et al. Time to onset and resolution of immune-related adverse events associated with ipilimumab therapy in patients with advanced melanoma. Proceedings of the 14th Perspectives in Melanoma. Amsterdam, The Netherlands, 2010:abstract P-0004.
- Lebbé C, O’Day SJ, Sileni VC, et al. Analysis of the onset and resolution of immune-related adverse events during treatment with ipilimumab in patients with metastatic melanoma. Proceedings of the 12th Perspectives in Melanoma. New York, NY, USA, 2008:abstract O-015.
- Lemech C, Arkenau HT. Novel treatments for metastatic cutaneous melanoma and the management of emergent toxicities. Clin Med Insights Oncol 2012;6:53-66. [PubMed]
- Rubin KM. Managing immune-related adverse events to ipilimumab: a nurse’s guide. Clin J Oncol Nurs 2012;16:E69-75. [Crossref] [PubMed]
- Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol 2010;21:409-14. [Crossref] [PubMed]
- Johnston RL, Lutzky J, Chodhry A, et al. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci 2009;54:2538-40. [Crossref] [PubMed]
- O'Day S, Weber JS, Wolchok JD, et al. Effectiveness of treatment guidance on diarrhea and colitis across ipilimumab studies. J Clin Oncol 2011;29:abstr 8554.
- Baurain JF, Smylie M, Ascierto PA, et al. Outcomes of ipilimumab treatment-related adverse events in patients with metastatic melanoma (MM) who received systemic corticosteroids in a phase III trial. J Clin Oncol 2012; 30:abstr 8539.
- Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 2013;98:1361-75. [Crossref] [PubMed]
- Ryder M, Callahan M, Postow MA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 2014;21:371-81. [Crossref] [PubMed]
- Kaehler KC, Egberts F, Lorigan P, et al. Anti-CTLA-4 therapy-related autoimmune hypophysitis in a melanoma patient. Melanoma Res 2009;19:333-4. [Crossref] [PubMed]
- Wilgenhof S, Neyns B. Anti-CTLA-4 antibody-induced Guillain-Barré syndrome in a melanoma patient. Ann Oncol 2011;22:991-3. [Crossref] [PubMed]
- Maur M, Tomasello C, Frassoldati A, et al. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J Clin Oncol 2012;30:e76-8. [Crossref] [PubMed]
- Bot I, Blank CU, Boogerd W, et al. Neurological immune-related adverse events of ipilimumab. Pract Neurol 2013;13:278-80. [Crossref] [PubMed]
- Papavasileiou E, Prasad S, Freitag SK, et al. Ipilimumab-induced Ocular and Orbital Inflammation-A Case Series and Review of the Literature. Ocul Immunol Inflamm 2016;24:140-6. [PubMed]
- Abdel-Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Ther Adv Respir Dis 2016;10:183-93. [Crossref] [PubMed]
- Abdel-Rahman O, Fouad M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy 2016;8:665-74. [Crossref] [PubMed]
- Kourie HR, Awada G, Awada AH. Rare side-effects of checkpoint inhibitors. Curr Opin Oncol 2016;28:295-305. [Crossref] [PubMed]
- Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210-25. [Crossref] [PubMed]
- Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N Engl J Med 2015;373:288-90. [Crossref] [PubMed]
- Nishino M, Chambers ES, Chong CR, et al. Anti-PD-1 Inhibitor-Related Pneumonitis in Non-Small Cell Lung Cancer. Cancer Immunol Res 2016;4:289-93. [Crossref] [PubMed]
- Kourie HR, Klastersky JA. Side-effects of checkpoint inhibitor-based combination therapy. Curr Opin Oncol 2016;28:306-13. [Crossref] [PubMed]
- Baroudjian B, Lourenco N, Pagès C, et al. Anti-PD1-induced collagenous colitis in a melanoma patient. Melanoma Res 2016;26:308-11. [Crossref] [PubMed]
- Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190-209. [Crossref] [PubMed]
- Márquez-Rodas I, Cerezuela P, Soria A, et al. Immune checkpoint inhibitors: therapeutic advances in melanoma. Ann Transl Med 2015;3:267. [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Callahan MK, Yang A, Tandon S, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol 2011;29:abstr 2505.
- Shahabi V, Berman D, Chasalow SD, et al. Gene expression profiling of whole blood in ipilimumab-treated patients for identification of potential biomarkers of immune-related gastrointestinal adverse events. J Transl Med 2013;11:75. [Crossref] [PubMed]


