Towards tumor immunodiagnostics
As beautifully communicated to children in the awarded 1990 “Cell Wars” book by F. R. Balkwill, immune cells are continuously alert for recognizing and destroying alien, non-self cells, as cancer cells are. But, cancers grow and spread, and the battle is lost for the host immune system and the host as a whole, if the host is left untreated. Are there any means to help the host regain the battle? Yes, and they have been proven efficient in previously considered as hopeless cases, but they still fail in others. Can we distinguish which tumors are easier to fight than others with or without standard treatment modifications?
The present review focuses on markers that are related to the tumor-host immune interplay status and dynamics, which can be assessed on routinely processed tumor tissue or cytologic material. Tumor immunodiagnostics is an emerging field in clinical practice involving the discovery, evaluation, validation and diagnostic application of markers that could provide actionable information to the clinician for patient assessment in all disease settings, i.e., pre- and post-therapeutically and upon disease progression or relapse. Thus, with respect to clinical relevance, potential immunodiagnostic markers are expected to be prognostic or predictive of response to classic cytotoxic treatments or immunomodulatory drugs.
Immunodiagnostic markers applicable on tissue or cytologic material may also be classified according to whether these are cell-specific or tumor-tissue-specific. Cell-specific markers are evaluated in situ under the microscope, almost always on formalin-fixed paraffin-embedded (FFPE) tissue sections. These are mostly distinguished into (I) histopathological, corresponding to the morphological assessment of immune cells on routine hematoxylin & eosin (H&E) sections; and (II) immunophenotypic, including the immunohistochemical (IHC) assessment of markers characteristic for tumor infiltrating immune cells. Tumor-tissue-specific markers are assessed in tissue extracts that may be enriched in neoplastic cells but almost inevitably also contain stromal and immune cells infiltrating the tumor. This class of markers may also be distinguished into two categories (I) immune-response-related gene expression profiles (immune GEPs); and (II) tumor genotype characteristics, as recently assessed with large-scale genotyping methods, usually next generation sequencing (NGS) applications.
After a brief historical and current concept review on our understanding of tumor-host interactions, the biological relevance of putative immunodiagnostic markers, their potential clinical relevance and the shortcomings that have as yet prevented their clinical application are discussed.
History and current concepts
In 1922, a pathologist and a surgeon reported that patients with removable breast “growths” with or without nodal involvement but with lymphocytic infiltrations in their tumors had a favorable outcome as compared to those without this feature; however, lymphocytic infiltrations alone did not appear to be the main factor affecting outcome (1). Similar reports followed in the next few years for cancer in other organs. Thirty years later, the issue of host-tumor relationship was discussed from the aspect of host-resistance against tumors (2). Fifty years later, all observations regarding immune cell infiltrates in tumors of all kinds were meticulously listed (3). The conclusion was that the immune infiltrates were there but they did not seem able to destruct tumor cells and that overall no causal relationship between this feature and the reported favorable outcome of the corresponding patients could be established. But the favorable outcome of patients with certain tumor types bearing tumor infiltrating lymphocytes (TILs) was a fact. Some 90 years later, tumor immunology was prophetically predicted as the key for anticancer treatment (4); the concept of immunoediting in cancer was re-shaped (5); “evading immune destruction” and “tumor promoting inflammation” were included in the revised version of the “hallmarks of cancer” (6); individual immune cells in the tumor microenvironment and their role in host-tumor interactions have been extensively described (7); key molecules (8,9) and drugs targeting these have been developed, successfully tested and introduced in the clinic (10), with cancer immunotherapy as the breakthrough of the year 2013 (11); and with immuno-oncology (and onco-immunology) starring in every international or local scientific cancer-related meeting.
The current concept of the host-against-tumor reaction is based on that genetic and epigenetic alterations in the neoplastic cells result in the generation of altered peptides, the neoantigens, which are perceived by the immune system as “non-self” and thus provoke an immune response (4,12). The tumor-host interaction introduces alterations in both the tumor cells and the immune system, a process described as immunoediting (13,14). These evolutionary changes occur through intricate pathways involving cellular and molecular components. Major cellular participants are helper T lymphocytes (Th1 and Th2), cytotoxic T lymphocytes (CTLs), regulatory T lymphocytes (Tregs), memory T lymphocytes, tumor-associated macrophages (TAMs), dendritic cells (DCs), natural killer cells (NK cells), B lymphocytes (B cells) and myeloid-derived suppressor cells (MDSCs). The type, density and location of the immune cells in the tumor microenvironment can be analyzed morphologically, via histopathologic examination, with the possible aid of image analysis systems. The above parameters, along with the functional status of the immune elements, as assessed by the secretion of cytokines or other mediators, have been referred to as the immune contexture (15). Tumor-associated molecular elements, such as neoantigens, enzymes e.g., indoleamine 2,3-dioxygenase (IDO), cytokines driving T-cell differentiation, tumor elimination or escape peptides, such as IFN-γ, IL-4, IL-10, and molecules involved in T cell or antigen-presenting cell (APC) suppression or activation, such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), lymphocyte activation gene-3 (LAG3) and toll-like receptor-4 (TLR4) are significant participants in the cancer and immune system cross-talk.
Histopathology of tumor infiltrating immune cells and structures: markers on H&E sections
TILs
On histopathologic examination, the tumor microenvironment contains various cell types of the innate and adaptive immunity. A prominent population consists of lymphocytes, called TILs. Melanoma was among the first tumors where the importance of TILs was noted (16), and nowadays the assessment of brisk, non-brisk or absent immune response is part of the standard parameters addressed in melanoma pathology reports (17). In various tumor types, the lymphoid cells, observed on H&E stain, are found scattered surrounding the tumor, called peritumoral, or within the tumor mass. The latter can either be identified in the stroma, between the epithelial cancer cell nests, and are termed stromal TILs, or infiltrating the tumor cell nests, in contact to the neoplastic cells, called intraepithelial or intratumoral TILs (18) (Figure 1A,B). The prognostic significance of TILs in breast carcinomas (19-21) led to an international proposal for their evaluation on H&E stained tumor sections (18). According to these recommendations, TILs are evaluated on H&E stained, whole tumor sections. Areas of average TIL infiltration within the neoplastic stroma are studied (Figure 1C) and the percentage of the stromal surface covered by TILs is recorded at 10% increments. Tumors with >50% lymphoid stromal infiltrates are characterized as lymphocyte-predominant breast carcinomas (18).
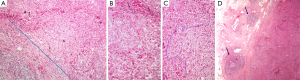
Tertiary lymphoid structures (TLSs)
The lymphoid cells infiltrating the tumor may be organized in TLSs, consisting of B and T cell compartments, mimicking the lymph node structure and function (22,23). The B cell areas show lymphoid follicles with or without germinal center formation (Figure 1D), including all the relevant constituents, namely, in addition to B cells at various stages of evolution, T follicular helper cells (Tfh), follicular dendritic cells (FDCs) and macrophages. The T cell areas show mature DCs and high endothelial venules. These structures are usually located at the periphery and beyond the confines of tumors, such as colorectal, breast or lung carcinomas. In the colon, they are usually noted around the muscularis propria or in the pericolic adipose tissue and because they are reminiscent of Crohn’s disease they are called Crohn’s-like reaction (CLR) (24,25). They are considered as tertiary lymphoid organs developing as part of the adaptive immune response to the tumor (25), are associated with prominent tumor infiltration by effector-memory CD8+ T cells and are considered important in educating CD8+ TILs in situ in order to control tumor growth (23,26,27).
H&E markers prognostic/predictive for disease outcome
The presence of TILs has been correlated with positive patient outcome in many tumor types, including colorectal cancer, melanoma, breast carcinoma, urinary bladder, prostate, renal cell, head and neck, lung, esophageal, gastric, pancreatic, hepatocellular, ovarian, endometrial and cervical carcinoma (15,28-31), although the prognostic significance of the various TILs subpopulations, TILs density and location may vary according to tumor type and stage (15,28,30).
During the last decade a significant body of evidence has accumulated regarding the importance of TILs in breast carcinoma. High TILs evaluated by H&E are more frequently observed in high grade, triple-negative breast carcinomas (TNBC) and HER2+ tumors, as well as in the prognostically favorable medullary carcinoma [reviewed in (32,33)]. Several studies evaluating the prognostic significance of TILs in breast carcinoma had contradictory results, possibly attributed to methodological variations. High TILs are associated with better prognosis in early stage TNBC in phase III trials with adjuvant anthracyclin-based chemotherapy (19,34,35), HER2+ breast carcinoma (36,37) and in lymphocyte-predominant breast carcinoma irrespectively of nodal status (36) but not in ER+ tumors (34). In accordance to these studies, a meta-analysis including 16,097 patients found that rich in TILs ER− breast carcinomas were associated with favorable prognosis (33).
High TILs were associated with response to neoadjuvant chemotherapy (38), in two meta-analyses including 3,251 patients (39) and 12,968 patients (40), in ER− breast carcinomas, according to a meta-analysis with 16,097 patients (33), in HER2+ breast carcinomas and TNBC (41). LPBC had a higher pCR than non-LPBC, particularly with the addition of carboplatin compared to the anthracyclin/taxane-only regimen (42). High TILs were also associated with response to adjuvant chemotherapy (34,36,38,43,44,). Furthermore, high TILs predicted benefit from trastuzumab therapy in the FinHER trial (35); in HER2-positive patients treated with trastuzumab, tumors with high TILs had the best outcome among all HER2-positive patient groups in a pooled analysis of four prospective clinical trials in the pre- and post-trastuzumab era (36); in this latter study, however, patients with low TILs also benefitted from trastuzumab as compared to those in the pre-trastuzumab era, and no interaction was demonstrated between TILs and this drug.
TLSs have been associated with favorable outcome in breast (45) and lung carcinomas (23). In the colon, TLSs and CLR along with prominent lymphocytic infiltration (≥3 intraepithelial/intratumoral lymphocytes per high power field) are considered as evidence of high microsatellite instability (MSI-H), associated with favorable outcome (46).
On the other hand and in addition to the T cell-inflamed tumor phenotype, where the neoplasm survives through immune-suppressive mechanisms, a non-inflamed tumor phenotype is also observed, where the immune system ignores the neoplasm (47). Recognition of the two distinct phenotypic immune tumor categories would potentially drive, in the future, distinct therapeutic approaches.
Phenotypic markers of tumor infiltrating immune cells: IHC markers
Markers per immune cell type and function
Several markers of the immune components are available for fresh or frozen tissues but these are more limited for FFPE tissue, which is the focus of the following discussion, since this is the type of material usually readily available. Of note, the same marker may label different cell types, while the same cell type may express more than one marker (Table 1).
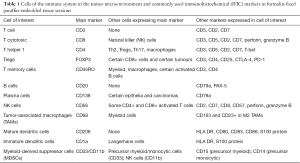
Full table
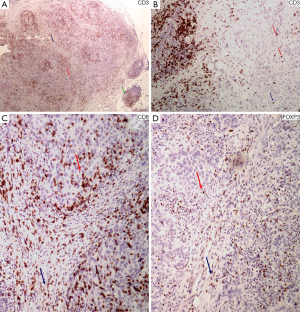
The vast majority of the lymphoid infiltrates in the tumor microenvironment consists of T lymphocytes. They express the pan-T cell marker protein CD3 (Figure 2A,B), which is associated with the T cell receptor (TCR) and is very specific for T cell derivation (31,48). The major T cell subtypes, the Th cells and CTLs can be recognized by the expression of the CD4 and CD8 molecules, respectively. These are both related to the TCR and aimed to recognize and bind to the MHC II molecule of the APC and the MHC I molecule of all nucleated cells, respectively, in order for the antigen recognition to occur by the T cell.
The acquisition of CD4 occurs in the thymus and is retained by the naïve CD4+ T cells that migrate to the secondary lymphoid organs, as well as by the various effector T cell subpopulations developing thereafter (49). Depending on the cytokine milieu at the site of the activation of the CD4+ naïve T cell, different subpopulations of CD4+ T cells will be produced. STAT-4 activation and the transcription factor T-bet drive the Th1 cell differentiation program and suppress the Th2 and Th17 cell differentiation. The Th1 lymphocytes are characterized primarily by the production of IFN-γ that induces CD8+ CTL maturation, NK cell activation and polarization of TAMs to the M1 anti-tumor subtype. These cell populations in concert exert a tumor elimination effect. Under the effect of IL-4, STAT-6 activation and the transcription factor GATA-3 drive the Th2 cell differentiation (49). Th2 lymphocytes produce primarily IL-4 and IL-5 and drive a humoral immune response and chemotaxis for eosinophils in the site of the tumor. Their role in tumor growth is unclear. CD4+ Th17 cells are characterized by the production of IL17 and their development is driven by STAT-3 and the retinoid acid orphan receptor (ROR)–γt (RORc) transcription factor under the influence of IL-6 and low concentrations of TGF-β (50). Th17 cells have been identified in several types of human cancer with contradicting results in patient outcome (51,52). CD4+ Tfh cells are present within the follicular germinal centers and in TLSs related to the tumors. They express the transcription factor BCL-6, PD-1, CXCR5 and molecules important for B cell development (49).
CTLs, identified by the expression of CD8+ (Figure 2C), recognize and kill cells bearing an antigen bound to a MHC I type molecule, which is present in all nucleated cells, including tumor cells (53). They result in target cell killing via the perforin and granzyme A/B system or through the expression of the FAS ligand. Both mechanisms result in induction of apoptosis in the target cell (54). Perforin and granzyme B (55) have been used as indices of an effector cytotoxic phenotype, although they are also seen in NK cells (48).
Tregs are suppressor cells with a beneficiary effect in regards to autoimmunity, allergy and transplant rejection but mixed effects in infection and cancer surveillance. Although Tregs comprise approximately 10% of total circulating CD4+ T cells, they amount up to 30–50% of CD4+ T cells in the tumor microenvironment, depending on the tumor type (56). There are two major Treg subpopulations, the natural Tregs (nTregs), produced in the thymus and involved in self-tolerance, and the induced or adaptive Tregs (iTregs), occurring in peripheral tissues and assisting in the adaptability and tolerance of the immune system towards microbial and tissue antigens (57). nTregs express high levels of CD25, the IL-2 receptor α chain, also present in lower levels on effector T cells. Development and function of nTregs depends on the expression of forkhead box P3 (Foxp3), a member of the forkhead-box/winged-helix transcription factors, that allows for their identification (58) (Figure 2D). Foxp3 is transiently expressed at lower levels by both CD4+ and CD8+ cells upon stimulation, although these cell types can be identified by the expression of IFN-γ, TNF-α and IL-2 (57). CD4+ CD25+ Foxp3+ nTregs suppress CD4+ and CD8+ T cell function, activation and proliferation, B cell proliferation and antibody production, NK cell activation and maturation of DCs, while skewing TAMs towards an M2 tumor-assisting phenotype. The tumor microenvironment is responsible for the generation of tumor-related iTregs, which are phenotypically similar to nTregs, expressing CD4, CD25 and Foxp3, although there are also subpopulations of iTregs that may not express Foxp3 (58). Tregs may also arise from CD8+ T cells, either intrathymically (nTregs) with a CD8+, CD25+, Foxp3+, CTLA-4+ phenotype, or at the periphery (iTregs), including tumor sites, with suppressive function through cell-cell interactions (57). Since an entirely specific and sensitive marker of Tregs is not available, additional markers expressed on Tregs have been investigated. These include the IL-2-related proteins CD122 και CD132 (59), CD127, the IL-7 α subunit (60), CTLA-4 (59), glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR), a costimulatory molecule involved in regulation of Treg cell function (60), OX40 (CD134) of the TNF receptor superfamily (61), PD-1, toll-like receptors (TLRs) and chemokine receptors CCR4 and CCR5 (56). Inducible co-simulator (ICOS), a CTLA-4 and CD28 homolog, related to Tfh development characterizes highly suppressive Tregs. HELIOS, a member of the “Icaros” family of transcription factors, is found on nTregs but not on iTregs, although it does not appear to represent a reliable marker for the distinction between the two groups (60). LAG-3/CD223, a CD4 homolog, is expressed on Tregs from cancer patients both at the tumor site and peripheral blood (58,59). Neuropilin-1 (Nrp-1) is also been considered as a Treg index marker (58).
Memory cells are characterized by the expression of CD45RO, although this marker is also localized on myeloid cells, macrophages and certain activated B cells and pre-plasma cells (62).
B cells can be reliably identified by the pan B cell marker CD20, recognized by the antibody L26, the immunoglobulin associated molecule CD79a, also expressed in plasma cells or the nuclear expression of the transcription factor PAX-5 (62,63).
Plasma cells are labelled by CD138, a very sensitive and specific marker for this cell type (48). The marker can also be expressed in certain epithelial cells and malignancies but the cell morphology between the populations is distinctive enough to prevent misinterpretation.
DCs function to present antigens and prime T and B cell immune responses to non-self antigens, as well as in maintaining tolerance. They express S-100 protein, a marker expressed in several other cells types and tumors, including melanoma, several types of epithelial cancers and nerve sheath-derived tumors. Mature DCs express CD208 dendritic cell lysosomal-associated membrane protein (DC-LAMP) (64) and immature DCs CD1a. Their functional status relies on their maturation status. Immature tumor infiltrating DCs (TIDCs) support tolerance of cancer cells, while mature TIDCs assist cancer elimination (65). Phenotypically mature DCs acquire expression of MHC class II, CD80, CD83 and CD86, these latter markers being negative or low on immature DCs. Recent data point to the presence of additional subtypes of semi-mature DCs with either functional (e.g., cytokine expression profile) or phenotypic disparity from the fully mature DCs, leading to the assumption that the initial dichotomous view of DCs as either immature/tolerogenic or mature/immunogenic may be obsolete (65). It has been suggested that DNRG expression by DCs is associated with an antitumor response while Foxp3 expression may be related to T cell tolerance (47).
NK cells, although traditionally considered to be a component of the innate immunity, they are also assisted in developing their full potential after priming by the DCs (66,67). CD56 expression is considered typical of NK cells, although this marker is present in subsets of CD4+ and CD8+ cells (48). They express NK cell receptors, such as NKp46 and NKG2D, as well as bright CD56. A CD56dim subpopulation of NK cells expresses CD57, a marker also shared by a subpopulation of T cells. The CD56dim/CD57 bright NK cells have an enzymatic and functional profile associated with high lytic capacity, thus they are considered to represent an effective antitumor response.
MDSCs are a heterogeneous population of myeloid progenitor cells exhibiting myeloid or monocytic differentiation and significant immunosuppressive function against tumors, mainly through direct inhibition of CTLs and NK cells. Human MDSCs express the myeloid markers CD33 and CD11b, are HLA-DR negative, with the myeloid and the monocytic precursors also expressing CD15 and CD14, respectively (68,69).
TAMs have a dual antigen-presenting and phagocytic function. They are positive for CD68, a lysosomal protein recognized by two antibody clones, KP1 and PGM1. The latter clone is considered more specific for histiocytic differentiation, while the former is also shared by myeloid cells. After exposure to LPS or IFN-γ they may be polarized to the M1 subtype, driving a Th1 immune response or frequently in the tumor microenvironment their polarization is skewed towards the M2 subtype positive for CD163, that drives a Th2 response, immunotolerance towards the tumor, assists tissue remodelling and tumor angiogenesis (62,70).
Prognostic individual IHC markers for disease outcome
The most extensively studied immune cell types are the CD3+ T cells, the CD8+ CTLs, the CD4+ T cells, the Foxp3+ Tregs and the CD45RO+ memory cells. In a meta-analysis evaluating the prognostic significance of intratumoral CD3+, CD4+, CD8+ and Foxp3+ TILs, in several tumor types including ovarian, colorectal, lung, hepatocellular and renal carcinomas among others, CD3+ and CD8+ TILs were associated with increased overall survival (OS) with hazard ratios (HR) of 0.58 and 0.71, respectively, and 95% confidence intervals (CI): 0.43–0.78 and 0.62–0.82, respectively. Although Foxp3+ TILs were not linked to OS, with HR 1.19 and 95% CI: 0.84–1.67, the ratio CD8/Foxp3 was strongly linked to OS (HR 0.48, 95% CI: 0.34–0.68) (71).
In a meta-analysis of TILs in colorectal cancer, Mei and colleagues found that high CD8+ and CD3+ TILs in the stroma of the invasive tumor front were associated with higher OS (HR 0.78 and 0.63, 95% CI: 0.67–0.82 and 0.42–0.93, respectively) (30). A meta-analysis of TILs in early, TNBCs noted that TIL-rich TNBCs were associated with better OS (HR 0.66, 95% CI: 0.53–0.83) irrespectively of TIL location (intratumoral or stromal) or mode of detection (H&E, CD8+ or Foxp3+ populations) (33).
CD8+ TILs have significant anti-neoplastic function, as indicated by their positive effect on prognosis of patients with melanoma, lung, breast, colorectal, cervical, endometrial, ovarian, urothelial, prostate, head and neck, esophageal, pancreatic and hepatocellular carcinomas (15,63,72). Similarly, memory CD45RO+ cells have been associated with higher disease-free survival (DFS) and/or OS in several cancer types (15,73), while the prognostic significance of B cells, Tregs, TH2, TAMs and MDSCs differs according to cancer type and stage (15).
CD8+ TILs have been associated with increased breast cancer specific survival in TNBC (74-76), HER2+ and ER+/HER2+ breast carcinomas (74) and in basal-like breast cancer but not in non-basal TNBC or in other intrinsic subtypes (77). CD8+ TILs have also been associated with response to adjuvant (74) and neoadjuvant anthracyclin+/−taxane-based chemotherapy (38).
Studies of non-small cell lung carcinoma (NSCLC) have highlighted the prognostic importance of TILs [reviewed in (78)]. Donnem and colleagues have shown that CD8+ stromal TILs had independent prognostic significance and added independent prognostic information within each NSCLC stage categories I–IIIA, with even stronger prognostic impact of stromal CD8+ TILs at the invasive margin, eluding to a TNM-immunoscore (Im) implementation in NSCLC (79). On the other hand, in a large study with 1,290 patients, prognostic significance of CD8+ TILs was identified only in squamous cell carcinomas (80). A recent meta-analysis, including 29 studies and 8,600 patients, identified that high levels of CD8+ TILs in the tumor stroma and tumor nests were associated with better OS (HR 0.76 and 0.80, 95% CI: 0.62–0.93 and 0.67–0.91, respectively). Similar results were observed for CD3+ TILs, with HR 0.65 and 0.66 and 95% CI: 0.50–0.84 and 0.45–0.97, respectively. CD4+ stromal TILs were also associated with better OS (HR 0.65, 95% CI: 0.46–0.91). On the contrary FOXP3+ stromal TILs were associated with worse progression-free survival (PFS) (HR 2.67, 95% CI: 1.74–4.08) (81). In addition to immunohistochemically-detected markers, TLSs detected in NSCLC have been associated with long-term survival (22). Intense lymphocytic infiltration (>50% stromal TILs, corresponding to lymphocyte-predominant breast carcinomas) was associated with longer OS, DFS and specific DFS in resected NSCLC treated with platinum-based chemotherapy. Intense infiltration was observed in 9% of the total study cases (discovery and validation sets). There were no differences with regards to histologic types (adenocarcinoma and squamous cell carcinoma) and no predictive effect was observed (82). Based on the experience from colorectal and breast carcinomas, a prospective multicenter study is planned for the evaluation of TILs in NSCLC (78).
Foxp3+ TILs have been associated with young age, high grade, ER-negative breast carcinomas, imparting a poor prognosis in ER+ tumors but a good prognosis in the HER2+/ER− subtype (83). Of interest, the tumor-promoting function of Tregs and MDSCs can be reduced by paclitaxel, as it selectively induces Treg apoptosis and impairs MDSC function by promoting their differentiation towards M1 TAMs and DCs (40). Nonetheless, the 2015 St Gallen consensus conference panel majority did not conclude that TILs have prognostic or predictive validity in breast carcinoma (84).
The role of Foxp3+ TILs in prognosis has been controversial. In their review deLeeuw and colleagues found that Foxp3+ TILs were associated with consistently poor prognosis in hepatocellular carcinoma and more frequently with poor prognosis in breast carcinoma. Neutral or good prognostic value was seen in colorectal, bladder, ovarian or oral carcinomas (85). A putative explanation of the paradoxically positive effect of Foxp3+ cells in colorectal carcinoma prognosis has been attributed to their inhibitory effect on the tumor-promoting Th17 cells. Th17 cells are accumulated in the microbe-rich environment of the bowel and colorectal carcinomas as an antimicrobial defence mechanism and produce tumor-promoting cytokines. It has been suggested that the inhibitory effect of Tregs on Th17 cells may explain the paradoxically positive role of Tregs on prognosis of colorectal carcinomas or tumors in sites with endogenous microbial-rich flora, such as the oral carcinomas (86). High numbers of NK cells were associated with improved prognosis in gastric, lung and colorectal carcinomas [reviewed in (67)].
Combinations of IHC markers and Im
Another approach in evaluating the effect of TILs on prognosis is through the combined analysis of markers, either as marker ratios or marker co-expressions.
The ratios most frequently evaluated, as identified by a meta-analysis of TILs in various tumors by Gooden et al., were CD8:FOXP3 and CD8:CD4 (71); the CD8:FOXP3 ratio was associated with increased OS in six studies (HR 0.48, 95% CI: 0.34–0.68), with increased DFS in two studies and PFS in two additional studies. The importance of this ratio is also exemplified in medullary breast carcinoma that is typically associated with favorable prognosis. This tumor type, although infiltrated by a significant number of FOXP3+ cells, showed a CD8:FOXP3 ratio of 2.6, compared to breast carcinomas of the non-specific type that had a ratio of 1.1 (87). High levels of CD8+ TILs and absent FOXP3 TILs after neoadjuvant chemotherapy were associated with complete pathologic response (88), while high CD8:FOXP3 in breast resection specimens after neoadjuvant chemotherapy was associated with improved recurrence-free survival (RFS) and OS (86) Other ratios that have been evaluated are CD3:CD8, FOXP3:CD3, FOXP3:CD4 and CD8:CD4, the latter examined in three studies and found to be significant in one (71).
Combination of markers, such as CD8+ and CD45RO+ TILs, have been shown to relate to good outcome in several tumor types including colorectal carcinoma (15). The combination of marker analysis was used in establishing the Im, a powerful prognostic tool originally evaluated in colorectal carcinomas (73,89). The Im takes into account the identification of TIL populations expressing CD3, CD8 and CD45RO. The markers are examined as pairs (CD3/CD8, CD3/CD45RO and CD8/CD45RO) in both the center and the invasive edge of stage I–III colonic adenocarcinomas. The expression of each marker in each tumor location could have a low or high value resulting in an Im ranging from Im0 (if all four values are low) to Im4 (if all four values are high). Tumors with Im4 are associated with longer DFS, disease specific survival (DSS) and OS, independently of stage. Thus, it was suggested that the Im has a significant value in the prediction of recurrence, independently of stage and clinicopathological parameters (55,90). An international study to evaluate the feasibility, reproducibility and prognostic value of the Im in colon carcinomas is been conducted and the markers to be evaluated are CD3 and CD8, given the high background staining seen with CD45RO and the granularity of granzyme B (28). Although the Im was originally introduced as a prognostic parameter, it has been proposed that it may function as a potential predictive marker for immune checkpoint targeting agents (27,28). The idea of implementing the Im in additional tumor types, including melanoma, breast, endometrial and ovarian carcinomas, was discussed in an international meeting of expert pathologists and immunologists (28), while strategies for the implementation of the Im in NSCLC have been proposed (78).
Predictive IHC markers for immunomodulatory drugs
CTLA-4 is expressed on T cells, in order to dampen the T cell activation responses after recognition by the T cell of an antigen presented by the APC. CTLA-4 ligands are expressed on APCs, but not on tumor cells and the resulting suppression is thought to occur in lymphoid organs and not in the tumor microenvironment (10).
PD-1 is also expressed on activated T and B cells, NKT cells, monocytes and Tregs and has a similar to CTLA-4 immunoregulatory function, aiming at controlling T cell activation. PD-L1 and PD-L2 are expressed on APCs but can also be expressed by many types of human cancers [reviewed by (91)] and upregulated by cytokines, such as IFN-γ and IL-4. The binding of PD-1 with PD-L1 results in inhibition of T cell activation, proliferation and cytokine secretion, resulting in immune evasion of the tumor cells in the tumor bed [reviewed by (92,93)], as well as an increase of Tregs in the tumor (94).
Cancer immunotherapy agents aim at CTLA-4 and/or PD-1 inhibition. Drugs targeting CTLA-4 are associated with global T cell activation and increase in tumor infiltration by T cells (91). Until now there is no predictive marker for CTLA-4 inhibitors (95), although high expression of FOXP3 and IDO at baseline biopsies and increases in TILs after initiation of treatment were related to increased clinical activity of ipilimumab in melanoma (96).
PD-L1 expression has been studied at the protein (97-102) or m-RNA (101-103) level in melanoma, lung, renal cell, bladder, ovarian, gastrointestinal, breast and head and neck carcinomas. In a meta-analysis of NSCLC studies, PD-L1 expression was associated with poor OS (HR 1.91, 95% CI: 1.33–2.95) (104). Agents blocking the PD-1/PD-L1 interaction, through binding of PD-1 or PD-L1, are used in melanoma, NSCLC, renal cell, bladder, head and neck, ovarian carcinomas and lymphomas (105,106) and progressively in more types of tumors. In several trials and tumor types, PD-L1 expression by the tumor or the immune cells has been used as a predictive marker, with tumors expressing PD-L1 displaying 48% response rates versus 15% in the PD-L1 negative tumors (107). In certain studies, PD-L1 expression has been associated with response to anti-PD-1 (108) or anti-PD-L1 agents (93) and in others PD-L1 expression by the lymphoid cells was predictive of clinical benefit (109). Thus, one major issue is the cell type expressing the protein, since the expression on antigen-presenting cells and lymphoid cells may be equally important for tumor containment, as is the expression on the neoplastic cells (106). There is significant heterogeneity regarding the IHC evaluation of PD-L1. The spatial heterogeneity (110) and temporal variation of PD-L1 expression within a given tumor or between tumor clones of a patient present a first obstacle (93). To complicate matters further, different therapeutic agents have as companion diagnostic tests different antibodies, platforms and methods of assessment (93,106). Different antibodies result in significantly discordant results (110). Varying IHC protocol conditions and platforms, staining patterns (membranous or cytoplasmic), thresholds for positivity (50%, 5% and 1%) and the inherent inter-observer variability of IHC studies result in remarkable variations in PD-L1 detection and assessment (111). Due to the lack of methodological standardization, definitive data on the utility of PD-L1 IHC evaluation as a predictive marker are not yet available and alternative methods, such as mutational profiles, are been investigated (112). On the other hand, an initiative to evaluate the reproducibility, prognostic and predictive value of the different PD-L1 assays for NSCLC is undertaken by the International Association for the Study of Lung Cancer (IASLC) (106). Similarly contradictory and inconclusive results are obtained regarding the prognostic role of PD-L1 (100).
Nevertheless, FDA approval has as yet (June 2016) been granted for three PD-L1 assays on routine diagnostic tissues with IHC, as companion diagnostics for selecting patients to receive an equal number of three therapeutic antibodies: the Dako mouse clone 22C3 for the anti-PD-1 pembrolizumab in the treatment of NSCLC (113) and melanoma; the Dako rabbit clone 28-8 for the anti-PD-1 nivolumab in melanoma; and, the Ventana clone SP142 for the anti-PD-L1 atezolizumab in bladder cancer. The true value of these tests in clinical practice will be evaluated in the years to come.
Shortcomings on IHC immune-cell related markers
Several problems may be encountered when assessing immune cell infiltration within a given tumor type or organ and among various tumor types. Tumors of various organs differ in their immunogenicity and the degree of immune cell infiltration and thus, are likely to be evaluated differently, e.g., melanoma and colon carcinoma are very immunogenic, while breast carcinoma is less so (99). On H&E assessment, examination of whole tissue sections will often reveal an uneven distribution of the inflammatory cells with variability between the center and the periphery of the tumor, or with focal or multifocal confluence of the immune cells, raising a question regarding the adequacy or inferiority of tissue microarrays (TMAs) for TILs evaluation, as well as the number, size and derivation of tissue cores for tumor representation. The spatial heterogeneity may introduce inter- or intra-observer variability in the quantification. The method of quantification/scoring, e.g., counting cells, estimating percentages, evaluating the tumor perimeter, or employing digital image analysis systems may also vary among tumor types, particularly with regards to the degree of the infiltration, e.g., colon carcinoma may be heavily infiltrated by lymphoid cells, rendering cell counting non-feasible and introducing the need for image analysis systems. The importance of intraepithelial, stromal or total TILs may vary among different tumors, e.g., for melanoma the significant TIL population consists of only those in direct contact with the melanoma cells (17), while in the breast the proposed mode of evaluation refers to stromal TILs, not touching the epithelial elements (37).
If cell subpopulations are to be examined by IHC, choice of the particular index markers, various antibody clones for each marker available for immunohistochemistry in FFPE tissue, assessment protocols and scoring modalities are also sources of variability (28). Certain index markers are remarkably robust and reliable, such as CD3 for detection of T cells, CD20/L26 for detection of B cells, CD138 for detection of plasma cells and CD8 for detection of CTLs. Others, such as CD4 used for Th cells, are not as specific, been observed in T cells with different functions, such as Tregs and Th17 cells, as well as in monocytic cells and DCs. FOXP3 is considered a relatively specific marker of Tregs, but it has been identified in activated T cells both effector and cytotoxic (60) and in neoplasms, among which breast carcinoma, hepatocellular carcinoma and melanoma (114). Furthermore, different antibody clones yield different staining patterns, raising concerns regarding antibody specificity. The intensity of the staining is at times an issue, e.g., CD25 is also considered a Treg marker but only at high levels of expression, since at lower levels it is observed on activated CD4+ and CD8+ effector T cells (60). Issues relating to PD-L1 reproducibility and applicability were discussed in the previous section.
Prognostic immune-related GEPs
As described, IHC is a widely applicable method that can eventually aid clinical decision-making when one or few markers are to be addressed, for a respective number of drugs. However, such a small number of molecular targets are generally not deemed adequate for assessing the functional program of a tumor and researchers have repeatedly turned to gene expression profiling by means of microarrays or qPCR for this purpose, such as Oncotype Dx, Mammaprint, Genomic Grade Index, Prosigna for breast (115) and the Oncotype Dx for colorectal cancer (116). In breast cancer, immune GEPs have been demonstrated as favorable predictors of response to neoadjuvant chemotherapy for all breast cancer subtypes (117) and as indicators of a distinct triple-negative breast cancer subtype that overlapped with GEPs from medullary breast carcinomas (118) that are lymphocyte rich, as described above, and carry excellent prognosis. However, none of these microarray GEPs, all of which had been determined in pre-existing microarray datasets, has been prospectively applied yet.
A different rationale with only a triplex RT-qPCR employing an endogenous reference and two target mRNAs has been also applied on FFPE tissues. Instead of the above-discussed CD3:CD8 IHC ratios for the mostly studied Im, CD3Z/CD8 mRNA profiles have been investigated in colorectal cancer (119). CD3Z and CD8 relative expression values have been clustered to create a score that was a favorable predictor of patient outcome, when both genes were highly expressed, much like the respective IHC Im. This triplex assay has recently been commercialized as ImmuneTYPER, but it has not yet received clearance for diagnostic use. Unlike the technically demanding and costly microarray platforms, especially for FFPE tissue templates, simple assays such as the one described may become clinically applicable if carefully validated.
Neoantigens, tumor mutational load and TILs
Recognizing the full profile of neo-antigens in a tumor demands extensive bioinformatics and HLA typing (12,120,121). Two basic aspects in this context are that the number of neo-antigens usually increases with increased mutational load in a tumor, however neo-antigens that mostly drive CD8+ cell activation come up to 92% from passenger mutations and only up to 8% from mutations in known oncogenes that are considered to drive the tumor; in comparison, CD4+ cell activation is almost exclusively driven by neo-antigens from passenger mutations (12).
Isolation of T cells that specifically react against neo-antigen epitopes produced by cancer cells was applied in a limited number of tumors and peripheral blood from patients with metastatic melanoma (122). Despite the fact that the rate of such cells in the periphery is very low their capture may be possible with current hyper-sensitive technologies. The approach may prove useful for personalizing immune therapy for cancer patients (123); however, the isolation and characterization of cells for neo-antigen specificity is still laborious and not applicable in routine diagnostics.
In comparison, the increasing spread of NGS applications for tumor genotyping may allow for the estimation of the tumor mutational load, which is related to the tumor neo-antigen load, and may be predictive of response to targeted immunomodulation (124) and probably to conventional chemotherapy, as well. Not all tumors carry similar numbers of mutations and tumors with a heavy impact of environmental carcinogens, such as melanoma and lung cancer, are among the heavily mutated ones (125). Increasingly, tumor classes with a dramatically high mutational load and distinct mutation patterns, are recognized to carry specific DNA repair defects, such as mismatch repair (MMR) gene defects previously known as tumors with a hypermutator phenotype (typically >1,000 mutations), within the Lynch syndrome or sporadic (126); BRCA1 mutations (127); APOBEC mutations (128); POLE mutations (129) the latter also characterized as ultramutated since they carry the highest mutational load among all tumor types, 100 s of 1,000 s.
The question with NGS applications is whether it is obligatory to use exome sequencing for assessing the entire coding mutations or whether inferring the rate of mutations that are obtained with targeted NGS panels would be sufficient. It seems that multigene panels are also suitable in predicting mutational load and MMR gene deficiency in the clinic (130). Of note, most of these 1,000 s of mutations in hypermutated tumors occur at very low frequency in the tumor DNA environment and it appears that sensitivity to checkpoint inhibitors is associated with the presence of clonal mutations that produce clonal neoantigens in tumors (131). Assessing the rate and clonal incidence of mutations with exome sequencing or, alternatively, with multigene panels may aid in selecting patients who will benefit from these treatments. These applications need to be standardized first and, although such assessments may become diagnostically applicable in the future, exome sequencing is still of limited use in the diagnostic context in general, and particularly so with respect to FFPE tissues which represent the bulk of diagnostic material. As discussed in the literature (124), further surrogate limited to cases with Lynch syndrome or with acquired MMR deficiency could be the investigation of MMR status by IHC, as regularly applied in pathology laboratories worldwide.
Further issues concerning tumor genotypes with NGS include the evolution of relapsed/metastatic tumor mutational status upon chemotherapy, which may be increased, and the acquisition of mutations in the immune response machinery, such as reported for deficient antigen presentation upon evolving mutations in HLA genes (132). Even in treatment naïve tumors, the mutational profiles attributed to tumor cells may also concern stromal cells or TILs. In the latter context, it was shown that leukocytes infiltrating breast carcinomas may carry mutations in known breast cancer genes or in leukemia associated genes that are not necessarily present in the germline (133). The gene encoding PD-L1 on chromosome 9 may be amplified in certain cancer types, e.g., cervical cancer, leading to over-expression of this protein (129). In fact, the mutational status of TILs has not yet been approached; however, these cells proliferate in situ in tumor tissues, which is positively related with their favorable impact on outcome (89); this function, however, may also be related to the acquisition of mutations in these non-neoplastic cells.
Conclusions
The interactions of neoplastic cells with the cellular and humoral immune components in the tumor microenvironment have been shown to be critical for the fate of the tumor, determining its survival or extinction. The immunoediting theory progression phases, namely elimination, equilibrium and escape, could possibly be reversed to the benefit of the patients, if the key features that drive this progression are dissected out. During the last decade, remarkable progress has been made in this field in basic, translational and clinical research. Major challenges in tumor immunodiagnostics are introduced by the variation of the immune response between organs and tumor types, by the heterogeneity of the distribution of the immune components, as well as by the great variety of available detection tools. It clearly appears that infiltration of tumors by CD8+ cytotoxic cells confers a positive prognostic value, while more complex methodologies, such as the Im, are likely to be included in the prognostic and predictive armamentarium, if validated by the ongoing clinical trials. Significant effort and progress is observed in colorectal, breast carcinoma and NSCLC that have been more extensively studied.
At present, the only immunodiagnostic markers that have entered clinical practice are the described FDA approved PD-L1 assays as companion diagnostics for checkpoint inhibitors. Further in situ markers, such as stromal and/or intraepithelial TILs assessment and Im are currently trialled for clinical applications by international consortia and a consensus (positive or negative) on their diagnostic use is awaited. Similarly, normalization and standardization of methods and tests for the assessments of the highly promising mutational load and mutation clonality are needed for the validation of such markers as predictive of response to established and newer immunomodulatory drugs.
Except for the above, a major issue that has progressively started to be discussed concerns the (re)appraisal of the continuous evolution of cancer cells but also of host cells including immune infiltrates within the same microenvironment, i.e., the tumor, and within the host as a whole. The status of DNA, which was previously considered as germline stable, is obviously dynamic and changeable. Based on the preliminary evidence mentioned for breast cancer, new questions arise: what if the observed mutational load does not only concern cancer cells but also stromal and immune cells? What if in parallel to cancer cells, the same or different mutational processes operate in hematopoietic cell progenitors? And, in a more generalized context, what is the mutational status of immune cells in chronic inflammation and autoimmune disease? If these questions are affirmatively answered, new paradigm shifts are expected in our view of cancer behavior and in our efforts to classify, type and treat cancers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sistrunk WE, Maccarty WC. Life expectancy following radical amputation for carcinoma of the breast: a clinical and pathologic study of 218 cases. Ann Surg 1922;75:61-9. [PubMed]
- Grace JT Jr, Kondo T. Investigations of host resistance in cancer patients. Ann Surg 1958;148:633-41. [Crossref] [PubMed]
- Underwood JC. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer 1974;30:538-48. [Crossref] [PubMed]
- Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest 2008;118:1991-2001. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Bindea G, Mlecnik B, Tosolini M, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013;39:782-95. [Crossref] [PubMed]
- Galon J, Angell HK, Bedognetti D, et al. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013;39:11-26. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450-61. [Crossref] [PubMed]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [Crossref] [PubMed]
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348:69-74. [Crossref] [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298-306. [Crossref] [PubMed]
- Elder DE, Guerry D 4th, VanHorn M, et al. The role of lymph node dissection for clinical stage I malignant melanoma of intermediate thickness (1.51-3.99 mm). Cancer 1985;56:413-8. [Crossref] [PubMed]
- Frishberg DP, Balch C, Balzer BL, et al. Protocol for the Examination of Specimens From Patients With Melanoma of the Skin. Available online: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-skin-melanoma-15protocol.pdf
- Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259-71. [Crossref] [PubMed]
- Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014;32:2959-66. [Crossref] [PubMed]
- Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology 2013;2:e24720. [Crossref] [PubMed]
- Loi S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol 2014;32:2935-7. [Crossref] [PubMed]
- Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008;26:4410-7. [Crossref] [PubMed]
- Remark R, Becker C, Gomez JE, et al. The non-small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med 2015;191:377-90. [Crossref] [PubMed]
- Graham DM, Appelman HD. Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol 1990;3:332-5. [PubMed]
- Kim JH, Kim KJ, Bae JM, et al. Comparative validation of assessment criteria for Crohn-like lymphoid reaction in colorectal carcinoma. J Clin Pathol 2015;68:22-8. [Crossref] [PubMed]
- Goc J, Germain C, Vo-Bourgais TK, et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014;74:705-15. [Crossref] [PubMed]
- Bremnes RM, Busund LT, Kilvær TL, et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J Thorac Oncol 2016;11:789-800. [Crossref] [PubMed]
- Galon J, Pagès F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med 2012;10:205. [Crossref] [PubMed]
- Quante M, Varga J, Wang TC, et al. The gastrointestinal tumor microenvironment. Gastroenterology 2013;145:63-78. [Crossref] [PubMed]
- Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014;110:1595-605. [Crossref] [PubMed]
- de la Cruz-Merino L, Barco-Sánchez A, Henao Carrasco F, et al. New Insights into the Role of the Immune Microenvironment in Breast Carcinoma. Clin Dev Immunol 2013. Available online: http://dx.doi.org/ [Crossref]
- Savas P, Salgado R, Denkert C, et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 2016;13:228-41. [Crossref] [PubMed]
- Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, et al. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat 2014;148:467-76. [Crossref] [PubMed]
- Loi S, Michiels S, Lambrechts D, et al. Somatic mutation profiling and associations with prognosis and trastuzumab benefit in early breast cancer. J Natl Cancer Inst 2013;105:960-7. [Crossref] [PubMed]
- Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014;25:1544-50. [Crossref] [PubMed]
- Kotoula V, Chatzopoulos K, Lakis S, et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget 2016;7:5074-87. [PubMed]
- Salgado R, Denkert C, Campbell C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol 2015;1:448-54. [Crossref] [PubMed]
- Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28:105-13. [Crossref] [PubMed]
- Mao Y, Qu Q, Zhang Y, et al. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One 2014;9:e115103. [Crossref] [PubMed]
- Yu X, Zhang Z, Wang Z, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol 2016;18:497-506. [Crossref] [PubMed]
- Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014;25:611-8. [Crossref] [PubMed]
- Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol 2015;33:983-91. [Crossref] [PubMed]
- Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 2012;132:793-805. [Crossref] [PubMed]
- West NR, Milne K, Truong PT, et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 2011;13:R126. [Crossref] [PubMed]
- Gu-Trantien C, Loi S, Garaud S, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013;123:2873-92. [Crossref] [PubMed]
- Tang LH, Berlin J, Branton P, et al. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum. Available online: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/cp-colon-16protocol-3400.pdf
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [Crossref] [PubMed]
- Gocke CD. Immunohistology of non-Hodgkin lymphoma. In: Dabbs DJ. editor. Diagnostic Immunohistochemistry. Churchill Livingstone Elsevier: Philadelphia, 2006:147.
- Dobrzanski MJ. Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front Oncol 2013;3:63. [Crossref] [PubMed]
- Abe M, Hiasa Y, Onji M. T helper 17 cells in autoimmune liver diseases. Clin Dev Immunol 2013;2013:607073.
- Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, et al. Immune system: a double-edged sword in cancer. Inflamm Res 2013;62:823-34. [Crossref] [PubMed]
- Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol 2013;182:10-20. [Crossref] [PubMed]
- Janeway CA, Jr, Travers P, Walport M, et al. Immunobiology, The Immune System in Health and Disease. 2th ed. New York: Garland Science, 2005.
- Weigelin B, Krause M, Friedl P. Cytotoxic T lymphocyte migration and effector function in the tumor microenvironment. Immunol Lett 2011;138:19-21. [Crossref] [PubMed]
- Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011;29:610-8. [Crossref] [PubMed]
- Tanchot C, Terme M, Pere H, et al. Tumor-infiltrating regulatory T cells: phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron 2013;6:147-57. [Crossref] [PubMed]
- Mougiakakos D, Choudhury A, Lladser A, et al. Regulatory T cells in cancer. Adv Cancer Res 2010;107:57-117. [Crossref] [PubMed]
- Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol 2013;4:190. [Crossref] [PubMed]
- Ménétrier-Caux C, Curiel T, Faget J, et al. Targeting regulatory T cells. Target Oncol 2012;7:15-28. [Crossref] [PubMed]
- Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther 2012;12:1383-97. [Crossref] [PubMed]
- Wainwright DA, Dey M, Chang A, et al. Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Front Immunol 2013;4:116. [Crossref] [PubMed]
- Knowles DM. Immunophenotypic markers useful in the diagnosis and classification. In: Knowles DM, editor. Neoplastic Hematopathology. 2nd ed. Philadelphia, PA: Lippincott Williams&Wilkins, 2001:93-226.
- Mahmoud SM, Lee AH, Paish EC, et al. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat 2012;132:545-53. [Crossref] [PubMed]
- Ozaki K, Nagata M, Suzuki M, et al. Isolation and characterization of a novel human lung-specific gene homologous to lysosomal membrane glycoproteins 1 and 2: significantly increased expression in cancers of various tissues. Cancer Res 1998;58:3499-503. [PubMed]
- Dueck AC, Reinholz MM, Geiger XJ, et al. Impact of c-MYC protein expression on outcome of patients with early-stage HER2+ breast cancer treated with adjuvant trastuzumab NCCTG (alliance) N9831. Clin Cancer Res 2013;19:5798-807. [Crossref] [PubMed]
- Lucas M, Schachterle W, Oberle K, et al. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 2007;26:503-17. [Crossref] [PubMed]
- Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun 2011;3:355-64. [Crossref] [PubMed]
- Katoh H, Watanabe M. Myeloid-Derived Suppressor Cells and Therapeutic Strategies in Cancer. Mediators Inflamm 2015;2015:159269.
- Jiang J, Guo W, Liang X. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Hum Immunol 2014;75:1128-37. [Crossref] [PubMed]
- Ugel S, De Sanctis F, Mandruzzato S, et al. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest 2015;125:3365-76. [Crossref] [PubMed]
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93-103. [Crossref] [PubMed]
- Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A 2007;104:3967-72. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 2014;25:1536-43. [Crossref] [PubMed]
- Chen Z, Chen X, Zhou E, et al. Intratumoral CD8+ cytotoxic lymphocyte is a favorable prognostic marker in node-negative breast cancer. PLoS One 2014;9:e95475. [Crossref] [PubMed]
- Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011;29:1949-55. [Crossref] [PubMed]
- Liu S, Lachapelle J, Leung S, et al. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 2012;14:R48. [Crossref] [PubMed]
- Donnem T, Kilvaer TK, Andersen S, et al. Strategies for clinical implementation of TNM-Immunoscore in resected nonsmall-cell lung cancer. Ann Oncol 2016;27:225-32. [Crossref] [PubMed]
- Donnem T, Hald SM, Paulsen EE, et al. Stromal CD8+ T-cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:2635-43. [Crossref] [PubMed]
- Ruffini E, Asioli S, Filosso PL, et al. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg 2009;87:365-71; discussion 371-2. [Crossref] [PubMed]
- Geng Y, Shao Y, He W, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem 2015;37:1560-71. [Crossref] [PubMed]
- Brambilla E, Le Teuff G, Marguet S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:1223-30. [Crossref] [PubMed]
- Liu S, Foulkes WD, Leung S, et al. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 2014;16:432. [Crossref] [PubMed]
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533-46. [Crossref] [PubMed]
- deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 2012;18:3022-9. [Crossref] [PubMed]
- Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 2011;224:389-400. [Crossref] [PubMed]
- Anz D, Eiber S, Scholz C, et al. In breast cancer, a high ratio of tumour-infiltrating intraepithelial CD8+ to FoxP3+ cells is characteristic for the medullary subtype. Histopathology 2011;59:965-74. [Crossref] [PubMed]
- Ladoire S, Arnould L, Apetoh L, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 2008;14:2413-20. [Crossref] [PubMed]
- Mlecnik B, Bindea G, Angell HK, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med 2014;6:228ra37. [Crossref] [PubMed]
- Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009;27:5944-51. [Crossref] [PubMed]
- Daud A. Current and Emerging Perspectives on Immunotherapy for Melanoma. Semin Oncol 2015;42 Suppl 3:S3-S11. [Crossref] [PubMed]
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 2015;27:39-46. [Crossref] [PubMed]
- Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol 2015;67:4-17. [Crossref] [PubMed]
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015-29. [Crossref] [PubMed]
- Teixidó C, González-Cao M, Karachaliou N, et al. Predictive factors for immunotherapy in melanoma. Ann Transl Med 2015;3:208. [PubMed]
- Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011;9:204. [Crossref] [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [Crossref] [PubMed]
- Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013;49:2233-42. [Crossref] [PubMed]
- Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology 2014;3:e29288. [Crossref] [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [Crossref] [PubMed]
- Darb-Esfahani S, Kunze CA, Kulbe H, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016;7:1486-99. [PubMed]
- Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 Expression and Associated Tumor-Infiltrating Lymphocytes in Laryngeal Squamous Cell Carcinoma. Clin Cancer Res 2016;22:704-13. [Crossref] [PubMed]
- Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014;20:2773-82. [Crossref] [PubMed]
- Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 2015;41:450-6. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Hansen AR, Siu LL. PD-L1 Testing in Cancer: Challenges in Companion Diagnostic Development. JAMA Oncol 2016;2:15-6. [Crossref] [PubMed]
- Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 2015;23:32-8. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:46-54. [Crossref] [PubMed]
- Bhaijee F, Anders RA. PD-L1 Expression as a Predictive Biomarker: Is Absence of Proof the Same as Proof of Absence? JAMA Oncol 2016;2:54-5. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Jørgensen JT. Companion diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert Rev Mol Diagn 2016;16:131-3. [Crossref] [PubMed]
- Martin F, Ladoire S, Mignot G, et al. Human FOXP3 and cancer. Oncogene 2010;29:4121-9. [Crossref] [PubMed]
- Győrffy B, Hatzis C, Sanft T, et al. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res 2015;17:11. [Crossref] [PubMed]
- You YN, Rustin RB, Sullivan JD. Oncotype DX(®) colon cancer assay for prediction of recurrence risk in patients with stage II and III colon cancer: A review of the evidence. Surg Oncol 2015;24:61-6. [Crossref] [PubMed]
- Ignatiadis M, Singhal SK, Desmedt C, et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J Clin Oncol 2012;30:1996-2004. [Crossref] [PubMed]
- Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Pentheroudakis G, Raptou G, Kotoula V, et al. Immune response gene expression in colorectal cancer carries distinct prognostic implications according to tissue, stage and site: a prospective retrospective translational study in the context of a hellenic cooperative oncology group randomised trial. PLoS One 2015;10:e0124612. [Crossref] [PubMed]
- Gubin MM, Artyomov MN, Mardis ER, et al. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest 2015;125:3413-21. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Cohen CJ, Gartner JJ, Horovitz-Fried M, et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J Clin Invest 2015;125:3981-91. [Crossref] [PubMed]
- Sathyanarayanan V, Neelapu SS. Cancer immunotherapy: Strategies for personalization and combinatorial approaches. Mol Oncol 2015;9:2043-53. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Nik-Zainal S, Alexandrov LB, Wedge DC, et al. Mutational processes molding the genomes of 21 breast cancers. Cell 2012;149:979-93. [Crossref] [PubMed]
- Taylor BJ, Nik-Zainal S, Wu YL, et al. DNA deaminases induce break-associated mutation showers with implication of APOBEC3B and 3A in breast cancer kataegis. Elife 2013;2:e00534. [Crossref] [PubMed]
- Howitt BE, Sun HH, Roemer MG, et al. Genetic Basis for PD-L1 Expression in Squamous Cell Carcinomas of the Cervix and Vulva. JAMA Oncol 2016;2:518-22. [Crossref] [PubMed]
- Stadler ZK, Battaglin F, Middha S, et al. Reliable Detection of Mismatch Repair Deficiency in Colorectal Cancers Using Mutational Load in Next-Generation Sequencing Panels. J Clin Oncol 2016;34:2141-7. [Crossref] [PubMed]
- McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463-9. [Crossref] [PubMed]
- Giannakis M, Mu XJ, Shukla SA, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Kleppe M, Comen E, Wen HY, et al. Somatic mutations in leukocytes infiltrating primary breast cancers. npj Breast Cancer 2015. [Crossref]


