Melanoma immunotherapy dominates the field
Introduction
The incidence of melanoma is increasing worldwide and despite early detection and appropriate management, the number of patients dying from metastatic disease continues to rise. According to the WHO, approximately 80% of all skin cancer-related deaths are attributed to melanoma, although it comprises only 5% of all skin cancers (1). The prognosis of advanced melanoma remains poor, with median survival ranging from 6 to 9 months with chemotherapy (2). Survival outcomes for patients with advanced disease vary depending on the number of adverse prognostic factors, such as visceral disease or brain metastases and elevated lactate dehydrogenase (LDH) levels (3-5). Although the 5-year survival for local disease is as high as 98%, it falls to 15% for patients with distant metastases (5). Despite extensive clinical research over the past decades, the treatment options for metastatic disease had been limited, with melanoma being considered as one of the most chemotherapy-resistant malignancies (6). Many agents had been investigated, but the efficacy of these treatments remained poor.
Metastatic melanoma: unmet medical needs
Until recently, and over the past 30 years, only dacarbazine, hydroxyurea and interleukin-2 (IL-2) had gained FDA approval for the treatment of metastatic melanoma. None have ever been tested in a randomized phase III trial against a control, or have been shown to prolong survival. Hydroxyurea was approved in 1967 producing a 10% response rate (RR) and was further tested in combination trials (7,8). Dacarbazine has been extensively used as single-agent chemotherapy and in combination with other chemotherapeutic agents or other biological response modifiers (9-16). It has been widely used in clinical trials and has remained the standard of care for comparing the efficacy of new regimens in most phase II and III trials (17-20).
IL-2 was the second (after interferon alfa) exogenous cytokine to show antitumor activity against melanoma. It plays a central role in immune regulation, particularly T-cell proliferation (21). IL-2 was approved by the FDA in 1998 for the treatment of adults with advanced metastatic melanoma. However, this regimen was never tested in phase III trials. Its highly toxic profile, requiring inpatient intensive care, together with the high cost did not allow the extensive use of this cytokine. The treatment has never been approved in Europe (22).
New era in immunotherapy
Immunomodulation: cytotoxic T lymphocyte associated antigen 4 blockade
The concept of modulating the host immune response to fight cancer is becoming increasingly more popular, as more regulatory pathways are being characterized.
CTLA-4 is a key element in immune tolerance and one of the main negative regulators of T-cell-mediated antitumor immune responses. This molecule serves as a natural braking mechanism for T-cell activation, allowing a return to homeostasis following an immune response (23). This was most profoundly demonstrated in CTLA-4 knockout mice that developed a massive lymphoproliferative disorder, leading to lymphocytic infiltration and destruction of major organs (24-26). CTLA-4 is a homologue of CD28 that functions as an inhibitory receptor for B7 costimulatory molecules expressed on mature APCs (27,28). Following T-cell activation, CTLA-4 cell-surface receptors are upregulated and successfully compete with CD28 for binding to B7, sending an inhibitory signal that down-regulates T-cell activation (29,30). This inhibitory signal affects downstream targets of CTLA-4 that include cytokine production by Th1 and Th2 cells (31) and key components of the cell cycle machinery (Cdk-4, Cdk-6 and cyclin D3) required for cell cycle progression (32,33). Therefore, it was hypothesized that blocking the interaction of B7 with CTLA-4 might enhance T-cell activation, leading to a more robust antitumor immune response.
Anti-CTLA-4 mAbs with a much greater affinity for CTLA-4 than B7 (competitive inhibition) were cloned and shown to inhibit the interaction of B7 and CTLA-4 (30). The inhibitory signal produced by CTLA-4 is therefore blocked and T-cell activation is enhanced. Based on preclinical data, clinical trials were initiated with two fully human anti-CTLA-4 mAbs, which have different pharmacokinetic and pharmacodynamic properties: tremelimumab and ipilimumab.
Tremelimumab
Tremelimumab (CP-675, 206; Pfizer Inc.) is a fully human IgG2 mAb directed against CTLA-4 (30). In an open-label, phase I, dose-escalation study, 39 patients with solid malignancies received an intravenous infusion of tremelimumab at 1 of 7 dose levels, ranging from 0.01 to 15 mg/kg (34). Among 29 patients with melanoma, toxicities were generally mild to moderate in severity and were dose related (34). Two patients (7%) had complete responses (CRs) by RECIST criteria, 2 (7%) experienced partial responses (PRs) and 4 patients (14%) had stable disease (SD) (34). Furthermore, objective responses were durable (ranging from 37 to 51+ months) (35) suggesting a memory T-cell response to tumor-associated antigens.
Subsequently, an open-label phase II trial was conducted in patients with advanced melanoma randomized to receive either 10 mg/kg tremelimumab monthly (n=44) or 15 mg/kg tremelimumab every 3 months (n=45) (36) Four (9%) of the patients from the first arm and 3 (7%) of the patients from the second arm had an objective response (CR/PR) (36). The 15 mg/kg Q3M regimen was associated with a lower incidence of grade 3–4 adverse events (AEs) (36) and was selected for further study. In the single arm, pivotal phase II clinical trial with central radiologic review, conducted in 251 patients with previously treated metastatic melanoma, the RR was 9.1% per investigator and 6.6% per independent radiologic review, so the study failed to reject the null hypothesis that the RR does not exceed 10% (37). In a randomized comparative phase III study, tremelimumab was evaluated against dacarbazine or temozolomide in 655 patients with advanced relapsed or refractory melanoma. The Data Safety Monitoring Board stopped the trial for futility at the planned second interim analysis, after 340 deaths had occurred (38). However, follow-up for survival was continued and the final study analysis was performed in October 2010, when 534 events (82%) had occurred. Median overall survival (OS) by intent-to-treat was 12.6 months (95% CI, 10.8–14.3) in the tremelimumab arm and 10.7 months (95% CI, 9.4–12.0) in the chemotherapy arm. Survival at 2 and 3 years was 26.4% and 20.7% in the tremellimumab arm and 22.7% and 17.0% in the chemotherapy arm, respectively. The probability of progression-free survival (PFS) at 6 months was similar in the two arms. The centrally monitored data collection for this clinical trial captured an approximate 16% use of ipilimumab, in the control arm, that may explain the differences in the results of this phase III trial and two positive phase III trials with ipilimumab (38). Tremelimumab is now developed by AstraZeneca-MedImmune.
Ipilimumab
Ipilimumab has been the first ever drug to receive FDA approval (March 2011) based on positive results in OS. It is also the first new agent, over the past 13 years that gained approval for the treatment of metastatic disease.
Ipilimumab (MDX-010; Medarex Inc./Bristol-Myers Squibb Co.) is an IgG1κ mAb against CTLA-4, with a serum half-life of approximately 12 days (39). Among HLA-A*0201+ patients with stage IV melanoma (n=56) treated with 3 mg/kg ipilimumab every 3 weeks (Q3W) or 3 mg/kg initially and then 1 mg/kg Q3W in combination with a gp100 peptide vaccine, the overall objective RR was 13% (2 CRs, 5 PRs) (40). Fourteen patients (25%) had grade 3/4 immune-mediated side effects including colitis, dermatitis, uveitis, enterocolitis, hepatitis and hypophysitis (40).
Ipilimumab’s approval was based on the improved survival demonstrated when compared with gp100 alone in patients with previously treated metastatic melanoma. In a second study, a confirmation of the prolongation of survival was shown in treatment naïve patients with metastatic melanoma. In this pivotal study, ipilimumab was administered alone or in combination with a gp100-peptide vaccine and was compared with administration of the gp100-peptide vaccine alone in HLA-A 0201 patients with stage III and IV melanoma, who had shown disease progression while on therapy for metastatic disease (41). Ipilimumab was administered at a dose of 3 mg/kg Q3W and for up to four treatments. The median OS for the patients that received ipilimumab or ipilimumab with vaccination was 10.1 and 10 months, respectively, whereas for those on vaccination alone it was 6.4 months. The 2- and 3-year survival rates were 25–30% for the ipilimumab-containing arms, values that are almost double than those seen in the vaccination arm. The effect of the antibody on the OS was independent of age, gender, disease stage, prior therapy with IL-2 and LDH levels. Later cumulative evidence also concludes that HLA status does not affect ipilimumab’s action (42).
Another phase III trial compared the combination of ipilimumab plus dacarbazine with dacarbazine alone. Ipilimumab was administered at a dose of 10 mg/kg as a first line treatment in 502 patients with previously untreated metastatic melanoma in a 1:1 ratio (43). Again, OS was statistically significant in favor of the ipilimumab-containing treatment (11.2 vs. 9.1 months). Durable objective responses were observed (median duration of best overall response 19.3 months in the ipilimumab-dacarbazine group vs. 8.1 months in the dacarbazine group). The 2- and 3-year estimated survival rates for the ipilimumab group were 20.8% and 28.5%, respectively, rates that do not differ from those seen with the 3 mg/kg dose. Prolonged survival was noted among some patients who were followed for up to 4 years. Dacarbazine and ipilimumab given as a combination were found to elevate liver enzymes and many of these patients were unable to complete all four administrations of the regimen. Further analysis of these data concluded that adding dacarbazine to ipilimumab did not improve results. The rates of gastrointestinal events with ipilimumab were lower than expected on the basis of prior studies.
Additional data will be needed to clarify whether 4 doses of ipilimumab are sufficient for clinical benefit or whether maintenance doses are necessary. Data from a published phase III trial, as well as from the randomized phase II dose-ranging study, indicate that ipilimumab is better tolerated at 3 mg/kg compared to 10 mg/kg and that a maintenance dosing may not be necessary for either regimen. A randomized trial of 3 vs. 10 mg/kg has completed accrual.
The FDA approved ipilimumab at a dose of 3 mg/kg administered at 3-week intervals and for a period of 3 months, as a first- and second-line therapy in metastatic melanoma. In Europe, the EMA has given approval only for those patients that have exhibited disease progression while on other systemic treatments (44).
Furthermore, as immunotherapeutic agents produce antitumor effects by inducing cancer specific immune responses or by modifying native immune processes, the clinical response patterns extend beyond those of cytotoxic agents and can manifest after an initial increase in tumor burden or the appearance of new lesions (progressive disease). Newly designated immune-related criteria were defined (45). These novel immune therapy response criteria proposed, based on the shared experience from community workshops and several investigators, were evaluated using data from ipilimumab phase II clinical trials in patients with advanced melanoma. For the immune-related response criteria, only index and measurable new lesions are taken into account, in contrast to conventional WHO criteria, which do not require the measurement of new lesions, nor do they include new lesion measurements in the characterization of evolving tumor burden. Across the phase II clinical trial program, four patterns of response to ipilimumab therapy were observed in patients with advanced melanoma. Two of the response patterns are captured with conventional response criteria: (I) response in baseline lesions-evident by week 12, with no new lesions and (II) “SD” (which in some patients was followed by a slow, steady decline in total tumor burden). The other two response patterns are new and involve: (III) responses after an initial increase in total tumor burden and (IV) a reduction in total tumor burden during or after the appearance of new lesion(s), at time points later than week 12.
Tumor responses to ipilimumab may not occur until the post-induction period of therapy and a confirmation is needed by a repeat, consecutive assessment no less than 4 weeks from the date of first documentation. This should only be considered for patients with stable performance status and without deterioration in laboratory values. Community oncologists should be aware that conventional response criteria may not adequately assess the activity of immunotherapeutic agents, because PD by initial radiographic evaluation does not reflect therapeutic failure, or that it may be necessary to biopsy new lesions that in fact may not represent progression; however, until now, immune related response criteria have not shown superiority over RECIST criteria in randomized control trials. In addition, special consideration should be given to immune related adverse events (irAEs), while established guidelines can be used to manage the majority of irAEs effectively (46). These AEs exhibit a characteristic pattern in their occurrence; skin-related irAEs are expected after 2 to 3 weeks, GI and hepatic AEs after 6 to 7 weeks and endocrinologic AEs only after an average of 9 weeks. Frequencies of dose-limiting ipilimumab-related irAEs increased with dose, but not the quality or type of irAEs. Currently, algorithms have been developed to facilitate the management of irAEs for oncology practice (47).
The safety and activity of ipilimumab in patients with brain metastases, a frequent cause of death for patients with melanoma, was investigated in a phase II study, which enrolled 72 patients in two parallel cohorts (48). Patients in cohort A (n=51) were neurologically asymptomatic and were not receiving corticosteroid treatment at study entry; those in cohort B (n=21) were symptomatic and on a stable dose of corticosteroids. The primary endpoint was the proportion of patients with disease control. After 12 weeks, nine patients in cohort A exhibited disease control (18%), as did one patient in cohort B (5%). When the brain alone was assessed, 12 patients in cohort A (24%) and 2 in cohort B (10%) achieved disease control. This phase II trial demonstrated that ipilimumab has activity in some patients with advanced melanoma and brain metastases, particularly when such metastases are small and asymptomatic.
A pooled analysis of OS data from multiple studies was performed to provide an estimate of long-term survival observed in ipilimumab treated patients with advanced melanoma (49). This consisted of 1,861 patients, from ten perspective and two retrospective studies of ipilimumab, including two phase III trials (primary analysis) and an additional study of 2,965 patients from an expanded access program. In the primary analysis, 1,257 patients were previously treated, 604 were treatment naïve, with the majority of patients receiving ipilimumab 3 mg/kg. The additional analysis of OS included data from patients enrolled in a US multicenter, open-label, expanded access treatment protocol (EAP, CA184-045). This cohort of patients initially received ipilimumab 10 mg/kg, however, the protocol was amended in March 2010 to administer ipilimumab 3 mg/kg (50). Expanded EAP data were excluded from the primary analysis, because of the incomplete collection of OS data, but were combined with the primary analysis cohort to assess the sensitivity of the primary analysis. Patients were observed for OS for up to 10 years in some studies. Among 1,861 patients, median OS was 11.4 months with a 3-year survival rate estimated to be 22% (26% for treatment naïve patients and 20% for previously treated patients). Ten percent of the patients were observed for at least 50 months, with a maximum follow-up time of 119 months. Including data from the expanded access program, median OS was 9.5 months, with a plateau at 21% in the survival curve beginning around year 3.
Currently, new trials focus on the potential advantages of combination therapies with ipilimumab (e.g., ipilimumab and bevacizumab, targeted therapy, chemotherapy, radiotherapy or radiosurgery, interferon), with such trials still accruing or recently closed and results awaited with interests (www.clinicaltrials.gov, accessed March 3, 2016) (51).
Next generation immunotherapy agents: anti-PD-1 antibodies
The programmed death 1 (PD-1) protein is another T-cell coinhibitory receptor, with a structure similar to that of CTLA-4, but with a distinct biologic function and ligand specificity (52,53). It has two known ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) (54-57). In contrast to the CTLA-4 ligands, CD80 (B7-1) and CD86 (B7-2), PD-L1 is selectively expressed on many tumors and on cells within the tumor microenvironment in response to inflammatory stimuli (58-61). Blockade of the interaction between PD-1 and PD-L1 potentiates immune responses in vitro and mediates preclinical antitumor activity (62).
BMS-936558 (nivolumab) (also known as MDX-1106 and ONO-4538) is a high-affinity, fully human, PD-L1-specific, IgG4 (S228P) monoclonal antibody that inhibits the binding of PD-L1 to both PD-1 and CD80.
The anti-tumor activity and safety of BMS-936558 was evaluated in a total of 296 patients with advanced melanoma, non-small-cell lung cancer, castration-resistant prostate cancer and renal-cell or colorectal cancer (63). Patients received anti-PD-1 antibody at a dose of 0.1 to 10.0 mg per kilogram of body weight every 2 weeks (Q2W). Response was assessed after each 8-week treatment cycle. Patients received up to 12 cycles, until disease progression or a complete response occurred (63). Grade 3 or 4 drug-related AEs occurred in 14% of the patients, consistent with immune-related causes. No maximum tolerated dose was defined. Among 236 patients, in whom response could be evaluated, there were 94 patients with melanoma and an objective RR of 28% was observed. Responses were durable. Updated results of the efficacy of nivolumab showed an overall response rate (ORR) of 31% in more than 100 patients with metastatic melanoma and grade 3 toxicity in 15%, comparing favorably with ipilimumab. Responses were highly durable in another cohort of 132 melanoma patients treated with nivolumab, with the ORR being 30% and 5% complete responders.
Similar preliminary data from the phase I trial of another anti-PD-1 agent, MK-3475 (lambrolizumab) showed efficacy in ipilimumab pretreated and naïve patients, with an overall response in the first 135 treated patients of 38%, according to the response evaluation criteria in solid tumor (RECIST, version 1.1) and with a 52% confirmed RR in the cohort of patients that received 10 mg/kg Q2W (64). In this phase I trial, lambrolizumab was administered IV at a dose of 10 mg/kg every 2 or 3 weeks or 2 mg/kg Q3W. Tumor responses were accessed every 12 weeks, with a median follow-up of 11 months. The median PFS was longer than 7 months and responses were durable in the majority of patients (at the time of the analysis in March 2013). Low-grade AEs were reported in more than 95% of the cases, including fatigue and asthenia, fever and chills, myalgias and headaches, whilst grade 3 or 4 drug-related AEs were seen in 13% of the patients. Skin disorders were reported in 21% and diarrhea in 20% of the patients, with the highest incidence of overall treated AEs in the cohort of patients that received 10 mg/kg Q2W. Treatment related pneumonitis and renal failure was reported in less than 5% of the patients.
In an open-label expansion cohort of the phase I trial, 173 patients with ipilimumab-refractory advanced melanoma were randomized to IV pembrolizumab (MK-3475 previously known as lambrolizumab) 2 mg/kg Q3W or 10 mg/kg Q3W (65). Primary endpoint was overall response. Previous treatment with BRAF or MEK inhibitors or both was required for patients with BRAF-mutant melanoma, there was no limitation on the number of previous treatments and no baseline screening for brain metastases was required. The median follow-up was 8 months at the time of the analysis (October 18, 2013), with 42% of the patients being still on treatment and the most common reason for discontinuation being disease-progression (59 of 173 patients). The ORR was 26% at both doses, with median response duration not reached in either dose groups. The survival analysis was updated in May 2014, with an overall estimated survival at 1 year of 58% and 63% for the 2 mg/kg and 10 mg/kg dose cohorts, respectively. Drug-related AEs occurred in 80% of the patients; however grade 3–4 in only 12%. As previously described, the most common drug-related AEs were fatigue, pruritus and rash. In a pooled analysis of this phase I trial presented at ASCO 2015, with long-term efficacy data for 655 patients (median follow-up of 21 months), no further safety concerns were raised, with an 80% all grades drug-related AEs, with no treatment-related deaths and a drug-related discontinuation rate of 4%; the overall response was 33%, with 8% complete responders and 1- and 2-year survival rates of 66% and 49%, respectively (66).
A randomized phase II study (KEYNOTE-002) assessed the efficacy of 2 pembrolizumab doses (2 or 10 mg/kg Q3W) vs. investigator’s choice chemotherapy (ICC) in 540 ipilimumab-refractory melanoma patients, previously treated with BRAF or MEK inhibitor or both if BRAF mutant (67). The primary endpoint was PFS. PFS was improved in patients assigned to the two doses of pembrolizumab (HR=0.57 and HR=0.50, respectively, P<0.001). Six-month PFS for the three arms was 34%, 38% and 16%, respectively, whilst mean PFS was 5.4 months, 5.8 months and 3.6 months, respectively,. OS data were immature and final OS was to be assessed after 370 deaths. The incidence of grade 3–4 treatment-related AEs was higher in the chemotherapy group (26% vs. 11–14%). These findings are consistent with the findings in the ipilimumab-pretreated population cohort in the KEYNOTE-001 study and established pembrolizimab as a new standard of care for the treatment of ipilimumab-refractory melanoma and taken together, they provide no evidence that one pembrolizumab dosing regimen is superior to another.
Nivolumab was also tested in patients with melanoma who had progressed after ipilimumab (68). In this randomized, controlled, open-label, phase III trial, 272 patients were allocated to nivolumab and 133 to investigator-choice chemotherapy. The objective response was 32% vs. 11%. Grade 3–4 drug-related AEs were fewer in the chemotherapy group. There were no treatment-related deaths. This second trial corroborates the findings of the KEYNOTE-002 and establishes the use of anti-PD-1 antibodies in an ipilimumab-refractory population.
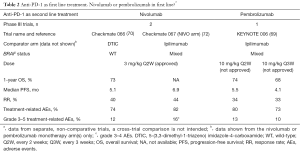
In the KEYNOTE-006 trial, pembrolizumab was compared with ipilimumab (69). BRAF V600 mutational status was required for enrollment. Previous BRAF inhibitor therapy was optional for patients with normal LDH and no clinically significant tumor-related symptoms or evidence of rapidly progressive disease (indolent disease). In this phase III trial, 834 patients were assigned in 1:1:1 ratio to receive pembrolizumab at a dose of 10 mg/kg Q2W or Q3W or the FDA approved regimen for ipilimumab. Primary endpoints were PFS and OS. The estimated 6-months PFS was 47% for the pembrolizumab arms and 26% for the ipilimumab arm (HR=0.58, P<0.001), whilst estimated 12-months survival was 74%, 68% and 58%, respectively. The RR was improved with pembrolizumab as compared to ipilimumab (33% vs. 11%, P<0.001 for both comparisons). This is the third trial to show a similar efficacy in two pembrolizumab regimens. Pembrolizumab had less high-grade toxicity than ipilimumab (grade 3–5 treatment-related AEs in the ipilimumab group was 19.9% (Table 1).

Full table
The promising results of anti-PD-1 antibodies in patients with ipilimumab refractory melanoma led to the design of a randomized phase III trial of nivolumab in previously untreated patients with advanced melanoma (70). Patients were eligible if they were BRAF wild-type (WT). This was a double blind study and the comparator arm was dacarbazine treatment, assigned in a 1:1 ratio. The primary end-point was OS. Approximately 410 patients were randomized. The trial was stopped early in June 2014, after the recommendation of the data and safety monitoring committee. This recommendation was based on an unplanned interim database lock that showed a significant difference in OS in favor of nivolumab. The study was unblinded and amended to allow patients enrolled in the dacarbazine group to cross over. The results from the double blind portion of the study (clinical data cutoff on June 24, 2014) showed that at 1 year the OS rate was 72.9% in the nivolumab group compared to 42.1% in the dacarbazine group (HR=0.42, P<0.001). The median PFS was 5.1 vs. 2.2 months, respectively (HR=0.43, P<0.001) and the objective RR was 40% vs. 13%, respectively (P<0.001). The improved OS in the nivolumab treated patients was regardless of PD-L1 status and was observed across pre-specified subgroups based on age, sex, metastasis stage, ECOG performance status score, status with respect to the history of brain metastases, baseline lactate dehyrogenase level and geographic region. The incidence of treatment-related AEs of any grade was similar in the two groups. However, serious AEs of grade 3 and 4 were less frequent in the nivolumab group (11.7% vs. 16.7%, respectively). The most common AEs related to the nivolumab treatment were as expected fatigue, pruritus and nausea, whereas in the dacarbazine group the toxic events were mostly gastrointestinal and hematologic.
Two additional studies in patients with metastatic melanoma who had not previously received treatment were conducted (71,72). Both studies built upon a phase I study, where concurrent therapy with nivolumab and ipilimumab was administered (73). The maximum tolerated dose was 1 mg/kg for nivolumab and 3 mg/kg for ipilimumab. In this phase I study, 53 patients received concurrent therapy and the objective response was 53%, all patients with tumor reduction of 80% or more. In this same study, 33 patients received sequenced treatment. Among patients in the sequenced regimen group, 18% had grade 3 or 4 AEs compared with 53% in the concurrent regimen group and the objective response was 20%. The clinical activity appeared to be distinct from that on monotherapy, with rapid and deep tumor regression. The OS at 1 year was 85% and at 2 years 79%.
The first study was a double blind, involving 142 patients randomly assigned in a 2:1 ratio to receive the combination or monotherapy with ipilimumab until the occurrence of disease progression or unacceptable toxicity. The primary end-point was the rate of investigator-assessed confirmed responses among patients with BRAF V600 WT tumors. The objective response was 61% with the combination vs. 11% in the ipilimumab montherapy group (P<0.001), with CRs in 22% vs. 0%, respectively. The median duration of response was not reached in either group. This was also the case for the median PFS with the combination therapy, while it was 4.4 months with the ipilimumab monotherapy group (HR=0.40, P<0.001). Similar results were observed in 33 patients with BRAF positive tumors. Overall, the characteristics of response observed with nivolumab plus ipilimumab were consistent with previously reported results, with most responses occurring by week 12 (time of the first tumor assessment). It should be noted that many patient responses continued to occur despite discontinuation of therapy. In addition, the RR associated with the combination regimen in this phase II study was higher than previously reported in the phase I trial, which might be explained by the fact that the patient population in this study was previously untreated. Drug-related AEs were reported in 54% of the patients who received the combination therapy; identical with the rate observed in the phase I trial, compared with 24% of the patients who received ipilimumab monotherapy.
The second was a randomized double-blind, phase III study of nivolumab monotherapy or combination of nivolumab with ipilimumab compared with ipilimumab alone in patients with metastatic melanoma. In total, 945 previously untreated patients were enrolled in a 1:1:1 ratio. PFS and OS were co-primary end-points. At a median follow-up ranging from 12.2 to 12.5 months across the three groups, the median PFS was 6.9 months in the nivolumab group, 11.5 months in the nivolumab plus ipilimuab group and 2.9 months in the ipilimumab group. The comparison between the nivolumab plus ipilimumab group and the ipilimumab group showed significantly longer PFS in the first group (HR=0.42, P<0.001); similar were the findings for the comparison between the nivolumab and ipilimumab groups (HR=0.57, P<0.001). Although the study was not designed for a formal statistical comparison between the nivolumab group and the combination group, the hazard ratio was 0.74. The combination resulted in a significantly longer PFS and a higher rate of response than nivolumab alone in the overall study population. As patients were stratified according to tumor PD-L1 status (positive vs. negative or intermediate), in patients with PD-L1 negative tumors PFS was longer with the combination therapy than with nivolumab alone (11.2 vs. 5.3 months), while the objective RRs were 54% vs. 41%. Grade 3 or 4 treatment-related AEs occurred in 55% of the patients in the combination group, 16% in the nivolumab group and 27% in the ipilimumab group (Table 2).
Full table
Talimogene laherparepvec (T-VEC)
Oncolytic viruses are novel cancer treatments that include WT and modified live viruses. T-VEC is a first-in-class oncolytic virus, based on a modified type I herpes simplex virus (HSV) designed to selectively replicate in and lyse tumor cells, while promoting regional and systemic antitumor immunity. T-VEC is modified through deletion of two nonessential viral genes (74). A randomized open label phase III trial was designed to compare T-VEC with GM-CSF in patients with unresected stage IIIB to IV melanoma (75). Patients were required to have injectable lesions that were not surgically resectable and were randomly assigned in a 2:1 ratio to intralesional T-VEC or subcutaneous GM-CSF. The primary end-point was durable response rate (DRR; objective response lasting continuously ≥6 months) per independent assessment. Secondary end-points included OS and ORR. In total, 436 patients were enrolled. DRR was significantly higher with T-VEC (16.3%) than GM-CSF (2.1%) (P<0.001). ORR was also higher in the T-VEC arm (26.4%). Median OS was 23.3 months with T-VEC and 18.9 months with GM-CSF (P=0.51). Subgroup analyses were performed and showed that differences in DRR between the T-VEC and GM-CSF arms were more pronounced in patients with stage IIIB or IIIC (33% vs. 0%) and IVM1a disease (16% vs. 2%) than in patients with stage IVM1b (3% vs. 4%) and IVM2c disease (7% vs. 3%). The same phenomenon was seen in patients with treatment naïve metastatic melanoma (24% vs. 0%). Treatment was well tolerated, with most common AEs being fatigue, chills and pyrexia. There were no treatment-related deaths. In October 2015, T-VEC received regulatory endorsements on each side of the Atlantic for its first-in-class oncolytic viral therapy.
Unanswered questions in 2016
With four immunotherapeutic agents in our armamentarium in 2016, the questions that remain to be answered are sequencing of treatments, combination with targeted agents, duration of treatment and biomarkers. Randomized control trials are under way and the results are awaited with interest.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- World Health Organization. Skin cancers. Accessed: June 4, 2010. Available online: http://www.who.int/uv/faq/skincancer/en/index1.html
- Garbe C, Eigentler TK, Keilholz U, et al. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 2011;16:5-24. [Crossref] [PubMed]
- Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest 2008;26:624-33. [Crossref] [PubMed]
- Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527-34. [Crossref] [PubMed]
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199-206. [Crossref] [PubMed]
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med 2004;351:998-1012. [Crossref] [PubMed]
- Carter RD, Krementz ET, Hill GJ 2nd, et al. DTIC (nsc-45388) and combination therapy for melanoma. I. Studies with DTIC, BCNU (NSC-409962), CCNU (NSC-79037), vincristine (NSC-67574), and hydroxyurea (NSC-32065). Cancer Treat Rep 1976;60:601-9. [PubMed]
- Hill GJ 2nd, Krementz ET, Hill HZ. Dimethyl triazeno imidazole carboxamide and combination therapy for melanoma. IV. Late results after complete response to chemotherapy (Central Oncology Group protocols 7130, 7131, and 7131A). Cancer 1984;53:1299-305. [Crossref] [PubMed]
- Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer 2004;40:1825-36. [Crossref] [PubMed]
- Fletcher WS, Green S, Fletcher JR, et al. Evaluation of cis-platinum and DTIC combination chemotherapy in disseminated melanoma. A Southwest Oncology Group Study. Am J Clin Oncol 1988;11:589-93. [Crossref] [PubMed]
- Vorobiof DA, Sarli R, Falkson G. Combination chemotherapy with dacarbazine and vindesine in the treatment of metastatic malignant melanoma. Combination chemotherapy with dacarbazine and vindesine in the treatment of metastatic malignant melanoma. Cancer Treat Rep 1986;70:927-8. [PubMed]
- Del Prete SA, Maurer LH, O'Donnell J, et al. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep 1984;68:1403-5. [PubMed]
- Legha SS, Ring S, Eton O, et al. Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol 1998;16:1752-9. [PubMed]
- Ridolfi R, Chiarion-Sileni V, Guida M, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol 2002;20:1600-7. [Crossref] [PubMed]
- Bajetta E, Del Vecchio M, Nova P, et al. Multicenter phase III randomized trial of polychemotherapy (CVD regimen) versus the same chemotherapy (CT) plus subcutaneous interleukin-2 and interferon-alpha2b in metastatic melanoma. Ann Oncol 2006;17:571-7. [Crossref] [PubMed]
- Eton O, Legha SS, Bedikian AY, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol 2002;20:2045-52. [Crossref] [PubMed]
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 2000;18:158-66. [PubMed]
- Buzaid AC, Legha SS, Winn R et al. Cisplatin (C), vinblastine (V), and dacarbazine (D) (CVD) versus dacarbazine alone in metastatic melanoma: Preliminary results of a phase II Cancer Community Oncology Program (CCOP) trial. Proc Am Soc Clin Oncol 1993;12:abstr 389a.
- Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999;17:2745-51. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [Crossref] [PubMed]
- Taniguchi T, Matsui H, Fujita T, et al. Structure and expression of a cloned cDNA for human interleukin-2. Nature 1983;302:305-10. [Crossref] [PubMed]
- Smith KA. Interleukin-2: inception, impact, and implications. Science 1988;240:1169-76. [Crossref] [PubMed]
- Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature 1987;328:267-70. [Crossref] [PubMed]
- Khattri R, Auger JA, Griffin MD, et al. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol 1999;16:5784-91. [PubMed]
- Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541-7. [Crossref] [PubMed]
- Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270:985-8. [Crossref] [PubMed]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65. [Crossref] [PubMed]
- Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005;12:1005-16. [Crossref] [PubMed]
- Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol 2005;23:8968-77. [Crossref] [PubMed]
- Alegre ML, Shiels H, Thompson CB, et al. Expression and function of CTLA-4 in Th1 and Th2 cells. J Immunol 1998;161:3347-56. [PubMed]
- McCoy KD, Le Gros G. The role of CTLA-4 in the regulation of T cell immune responses. Immunol Cell Biol 1999;77:1-10. [Crossref] [PubMed]
- Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 2002;16:23-35. [Crossref] [PubMed]
- Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol 2002;3:611-8. [Crossref] [PubMed]
- Gomez-Navarro J, Sharma A, Bozon V, et al, Dose and schedule selection for the anti-CTLA-4 monoclonal antibody (mAb) CP-675, 206 in patients (pts) with metastatic melanoma. J Clin Oncol 2006;24:abstr 8032.
- Bulanhagui CA, Ribas A, Pavlov D, et al. Phase I clinical trials of ticilimumab: Tumor responses are sufficient but not necessary for prolonged survival. J Clin Oncol 2006;24:abstr 8036.
- Ribas A, Antonia S, Sosman J, et al. Results of a phase II clinical trial of 2 doses and schedules of CP-675,206, an anti-CTLA4 monoclonal antibody, in patients (pts) with advanced melanoma. J Clin Oncol 2007;25:abstr 3000.
- Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 2010;16:1042-8. [Crossref] [PubMed]
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22. [Crossref] [PubMed]
- Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007;13:1810-5. [Crossref] [PubMed]
- Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005;23:6043-53. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Wolchok JD, Weber JS, Hamid O, et al. Ipilimumab efficacy and safety in patients with advanced melanoma: a retrospective analysis of HLA subtype from four trials. Cancer Immun 2010;10:9. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [Crossref] [PubMed]
- Eggermont AM, Robert C. New drugs in melanoma: it's a whole new world. Eur J Cancer 2011;47:2150-7. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691-7. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Yervoy (ipilimumab): Risk Evaluation and Mitigation Strategy (REMS) - Severe Immune-Mediated Adverse Reactions. Available online: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm249770.htm
- Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012;13:459-65. [Crossref] [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [Crossref] [PubMed]
- Hamid O, Hwu WJ, Richards JM, et al. Ipilimumab (Ipi) expanded access program (EAP) for patients (pts) with stage III/IV melanoma: 10 mg/kg cohort interim results. J Clin Oncol 2012;30:abstr 8508.
- ClinicalTrials.gov. Available online: www.clinicaltrials.gov
- Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141-51. [Crossref] [PubMed]
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 2007;19:813-24. [Crossref] [PubMed]
- Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193:839-46. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002;99:12293-7. [Crossref] [PubMed]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [Crossref] [PubMed]
- Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562-7. [Crossref] [PubMed]
- Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009;10:1185-92. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Daud A, Ribas A, Robert C, et al. Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. J Clin Oncol 2015;33:abstr 9005.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [Crossref] [PubMed]
- Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 2006;12:6737-47. [Crossref] [PubMed]
- Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]


