Superior sulcus tumors (Pancoast tumors)
Introduction
The term Pancoast or superior sulcus tumor defines a wide range of tumors invading the apical chest wall and producing a characteristic syndrome named “Pancoast-Tobias syndrome”. The Pancoast-Tobias syndrome involves severe and unrelenting shoulder and arm pain along with the distribution of the eighth cervical and first and second thoracic nerve trunks, Horner’s syndrome (ptosis, miosis, and anhidrosis), and atrophy of the intrinsic hand muscles. The unique characteristic of Pancoast tumors lies in the anatomy of the region where these tumors occur. For this reason, the surgical approach to these tumors is technically demanding and a complete resection may be difficult to accomplish. The treatment of Pancoast tumors has evolved greatly during the years: initially thought to be inoperable, the first case of surgical removal was reported in 1956 by Chardack and MacCallum (1). Since then, various authors published cases of successful surgical resection in association with radiotherapy. This has made the radio-surgical approach the treatment of choice for many years. In 1990s, induction chemo-radiotherapy followed by radical surgical resection was introduced as a new standard treatment for superior sulcus tumors. The treatment brought in impressively improved outcomes, and it still represents the gold standard today.
Historical background and definitions
In 1838, Hare (2) described the first recorded case of “tumor involving certain nerves”, producing constant and characteristic pain in the shoulder and the arm. However, it was Henry K. Pancoast in 1924 (3), studying radiographic findings of several cases, who first described this clinical entity of “apical chest tumors”, characterized by “small homogeneous shadows at the extreme apex”, “more or less” rib destruction and often vertebral infiltration. These tumors were described as associated with a clinical syndrome of pain in the distribution of the eighth cervical, first and second thoracic nerve trunks and Horner’s syndrome. In 1932, Pancoast published a second report (4), in which he defined them as “superior pulmonary sulcus tumors” and stated that they probably arose from epithelial rest cells from the fifth brachial cleft. The first to propose a pulmonary origin for these tumors was Tobías (5), who better defined the anatomical and clinical aspects of this syndrome. The Pancoast-Tobías syndrome is characterized by one or more of the following symptoms (6):
- Severe shoulder pain that aggravates over time. The pain may radiate to the neck, to the axilla, to the anterior chest wall, to the medial aspect of the arm and forearm as far as the wrist, along with the distribution of the ulnar nerve (C8–T1 nerve roots). The pain is provoked by the invasion of the chest wall and brachial plexus;
- Claude-Bernard-Horner syndrome (ptosis, miosis, enophthalmos and anhidrosis of the ipsilateral side of face), related to invasion of the sympathetic chain and of the inferior cervical (stellate) ganglion;
- Weakness and muscle atrophy of the intrinsic muscles of the hand, indicative of tumor infiltration of the ulnar nerve;
- Upper arm edema, in case of invasion and partial or complete occlusion of the subclavian vein.
A wide variety of diseases can cause Pancoast-Tobías syndrome: primary neoplasms of lung and pleura, metastases from other organs, hematologic malignancies, inflammatory and infectious diseases (7). The term Pancoast Tumor includes, however, only patients with a primary lung cancer in the typical location of the apex of the lung, regardless of whether any symptoms of the syndrome are present. The formal definition of Pancoast tumor, as provided by the American College of Chest Physicians (ACCP) (8), is: “a lung cancer arising in the apex of the lung that involves structures of the apical chest wall. Invasion of apical chest wall structures is required at the level of the first rib or above, but it is not necessary to have Horner syndrome or pain radiating down the arm. These lesions frequently invade the brachial plexus, subclavian vessels, or spine.”
Etiology and biology
The vast majority of Pancoast tumors are bronchogenic carcinomas, with adenocarcinoma as the most frequent histologic type. Small-cell carcinoma is only rarely associated with this syndrome. Less than 5% of all bronchogenic carcinomas evolve into Pancoast tumors (9). Initially Pancoast tumors seemed to have different biology from that of other non-small cell lung cancers (NSCLC), with higher propensity for local invasion and diminished incidence of spread through lymphatic or haematogenous lines (10). However, as pointed out by Detterbeck (11), the rates of pathologic N2 involvement in resected patients are not any different from those of other clinically stage matched NSCLC, and survival rates are better when lobectomy is performed rather than wedge resection. Thus, “the unique feature of Pancoast tumors appears not to lie in the tumor biology, but rather in the anatomy of the region in which these tumors occur”.
Anatomy
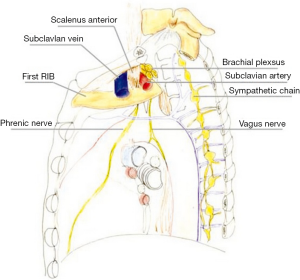
The precise anatomic definition of superior pulmonary sulcus is obscure, as most anatomy textbooks do not include it as a defined anatomic area. According to the description provided by Pancoast (4) the superior sulcus corresponds to the most cephalad extent of the costovertebral gutter. Other authors suggest the superior sulcus is formed by the subclavian artery as it crosses the pulmonary apex (12,13). Netter (14), proposed that the superior pulmonary sulcus actually “does not correspond to any recognized anatomic location” leading to the disuse of this term. The anatomy of the area affected by Pancoast tumors is rather based upon the description of the thoracic inlet and its subdivision into three compartments (15). The first rib forms the base of the thoracic inlet (Figure 1).
Anterior compartment (Figure 2A)
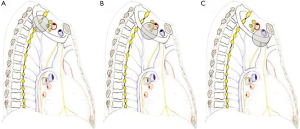
It is delimitated anteriorly by the sternum and posteriorly by the attachment of anterior scalene muscle on the first rib. It contains sternocleidomastoid and omohyoid muscles, subclavian and jugular veins, and scalene fat pad.
Middle compartment (Figure 2B)
It is located between the anterior and middle scalene muscles; subclavian artery, phrenic nerve, and trunks of the brachial plexus cross it.
Posterior compartment (Figure 2C)
It lies behind the middle scalene muscle. It contains the posterior scalene muscle, posterior scapular artery, the stellate ganglion, sympathetic chain, long thoracic and accessory nerves, neural foramina, and vertebral bodies. The invasion of this complex anatomical area accounts for the classic symptoms of Pancoast tumors.
Clinical presentation and diagnosis
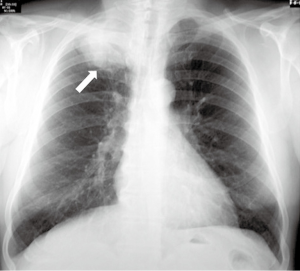
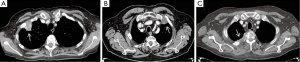
Due to the fact that the thoracic inlet is a narrow compartment, a modest growth and direct extension of the tumor will produce the aforementioned symptoms. Cough, hemoptysis, and dyspnea are uncommon during the initial stages of the disease, due to the peripheral location of these tumors (9,11). (I) Shoulder pain is the most common initial symptom; this leads to consultation with multiple non-thoracic specialists. As the syndrome progresses, there may be (II) paresthesia and (III) weakness in the ulnar nerve distribution and intrinsic hand musculature. (V) Horner’s syndrome is present in 15–50% of patients (9). Chest radiography (Figure 3) may show a mass or a pleural thickening in the apex of the lung, associated or not with invasion/destruction of the ribs and adjacent vertebrae. However, the tumor can easily be missed on chest X-rays. Computed tomography (CT) of the chest (Figure 4) will confirm the presence of an apical mass and its relationships with the bony thorax and with the other structures of the thoracic inlet. Magnetic resonance imaging (MRI) is recommended for better characterization of suspected brachial plexus, subclavian vessels, spine, and neural foramina invasion, because it can achieve a more precise assessment of the local extent of the disease and of the amount of nerve-root involvement (15). Given the peripheral location of the disease, the most sensitive procedure to obtain a tissue diagnosis is a percutaneous transthoracic needle biopsy, while biopsies obtained by fiberoptic bronchoscopy have a low diagnostic yield (9). When the above-mentioned methods are inconclusive, considering that a histological diagnosis is mandatory before starting any treatment, a video-assisted thoracoscopy or even minithoracotomy might be required (7).
Staging
By definition Pancoast tumors are classified as T3 tumors when they invade only the chest wall and/or the sympathetic chain. Tumors that invade the brachial plexus, vertebral bodies, and vascular structures are classified as T4. According to their N status, the final stage is IIB if the tumor is T3N0, IIIA if T3N1–2 or T4N0–1 and IIIB if T3N3 or T4N2–3 (16). A careful mediastinal staging is necessary as pN2 or pN3 involvement in Pancoast patients represents a particularly poor prognostic factor (17). Chest-CT and positron emission tomography-computed tomography (PET-CT) scans are mandatory before and after preoperative therapy, while no unequivocal recommendation can be made about whether mediastinoscopy or endobronchial ultrasound/esophageal ultrasound should be performed (8). Supraclavicular ipsilateral lymph nodes deserve a special mention because they seem to be involved in Pancoast tumors more frequently than in other NSCLC (17). It is a matter of debate whether positive lymph nodes at this level represent N3 metastatic spread or rather a direct involvement for local extension, given the contiguity of this area to the thoracic inlet. According to this hypothesis, supraclavicular lymph node involvement would result in a better prognosis than the presence of other mediastinal N2 or other N3 stations. Given the locally advanced extent of the disease, a careful search should be made for clinically silent distant metastases, especially in the brain (15). The ACCP (8) guidelines recommend to perform brain CT or MRI in association with either whole-body PET-CT or abdominal CT plus bone scan.
Treatment and survival
The particular growth features of the Pancoast tumor have affected the treatment regimen since its disclosure. In the early years (1930s–1950s), Pancoast tumor was recognized as a singular clinical entity and considered inoperable and incurable. In 1932, Pancoast stated “… [Superior sulcus tumor] resisted all efforts at irradiation treatment, it is obviously not subject to surgical removal although it is accessible, and it is rather rapidly fatal” (4). In the following years, Walker (18) and Herbut and colleagues (19) reported their experience with irradiation in superior sulcus tumors, although their reports didn’t show any substantial benefit, even in a palliative setting. The first reported cure of a superior sulcus tumor was attempted only in the fifties: Chardack and MacCallum (20) performed a surgical resection followed by postoperative radiation. The patient was alive and disease-free 5 years later. In 1956, Shaw (10) introduced a new treatment paradigm: he reported a case with typical Pancoast syndrome that initially underwent radiotherapy with a palliative intention, which resulted in resolution of pain and decrease in tumor size, and therefore it was followed by a radical resection. Since then, bimodality therapy (radiotherapy plus surgery, through postero-lateral approach) has become the standard of care. A large number of series (17,21-25) with this bimodality approach have been published, reporting similar survival (between 26% and 35% at 5 years) and complete resection rates (±60%). These survival and resection rates remained poor and unchanged over the subsequent 40 years. Surgical resection continued to be limited only to tumors invading the ribs. Any involvement of vascular or neural structures was considered a contraindication to surgery (26). In the Eighties, novel surgical approaches were introduced for the resection of tumors involving the spine and subclavian vessels. Dartevelle et al. (23) developed an anterior transcervical approach for cancers infiltrating the anterior part of the inlet and the subclavian vessels. Subsequently, several modifications of this technique were reported (26-28). Radical surgery with acceptable local tumor control became a real possibility thanks to new surgical techniques such as laminectomy, partial or hemivertebrectomy, complete resection of vertebral body, and vascular by-pass, as well as the common practice of working in a multidisciplinary team (thoracic surgeon, neurosurgeon, orthopedic surgeon, vascular surgeon) (24,29-33). During the 1990s, the increasing experience with combined modality therapy suggested that induction chemo-radiotherapy followed by resection could become an effective treatment strategy for stage III NSCLC (34). These new findings, associated with the need to improve systemic as well as local control, acted as a base for the development of two trials for Pancoast tumor treatment: the Southwest Oncology Group (SWOG 9416) prospective, multi-institutional, phase II trial (35) and the Japan Clinical Oncology group (JCO 9806) phase II trial (36). In both studies, improvements in completeness of resection and pathological response rates to chemo-radiotherapy were reported. Even long-term (5-year) survival rates resulted improved reaching 44% (SWOG 9416) and 56% (JCO 9806), respectively. Based on these data, the modern standard treatment for sulcus superior tumors has become the combination of induction CT/RT followed by radical surgical resection (25,29,35-43) (Table 1).
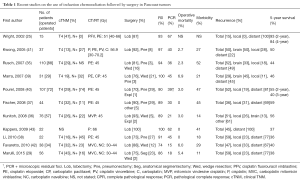
Full table
Surgical approaches
The complex anatomy of the thoracic inlet led to the development of various surgical approaches to properly expose the tumor and the involved structures.
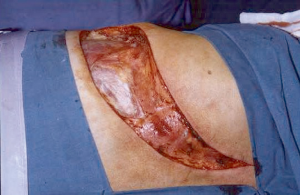
High postero-lateral approach (Figure 5)
This was the first surgical approach described by Shaw and Paulson (10). The incision extends around the tip of the scapula and reaches midway between the posterior edge of the scapula and the spinous processes, up to the level of C7. Posterior thoracotomy allows a wide exposure of the posterior chest wall, transverse processes, vertebrae and roots of the thoracic nerves and the plexus.
Anterior transcervical-thoracic approach
This approach was described and popularized by Dartevelle et al. (23). The incision runs along the anterior border of the sternocleidomastoid muscle and continues laterally above the clavicle, the medial portion of the clavicle has to be excised. This anterior approach allows an excellent exposure of the entire thoracic inlet and particularly of the subclavian vessels and brachial plexus. Transection of the clavicle, however, causes postoperative alterations in the shoulder anatomy, mobility and cervical posture (44).
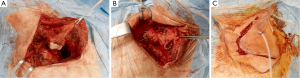
Transmanubrial L-shaped approach (Figure 6). Grunenwald and colleagues (27) developed this technique to overcome the superior girdle instability due to the clavicle transection in the Dartevelle approach. The transclavicular incision evolved into a transmanubrial incision preserving the integrity of the clavicle and its muscular insertions and raising both as an osteomuscular flap.
Anterior trans-sternal approach
Introduced in 1979 by Masaoka (45), it consists of an upper median sternotomy with an extension into the anterior fourth intercostal space, and a transverse incision above the clavicle at the base of the neck. It provides excellent exposure of the anterior upper chest wall, particularly when the tumor involves subclavian artery, brachial plexus or the superior vena cava.
Hemiclamshell or trapdoor approach (28)
This incision includes a partial sternotomy prolonged into an anterior thoracotomy usually in the fourth intercostal space. It allows the exposure of the same structures as in the Masaoka approach.
In the event of a complex superior sulcus tumor with the involvement of anterior and posterior inlet structures, a combination of the two surgical accesses could be used (i.e., anterior approach and thoracotomy). A double incision could allow a radical tumor resection but may increase the complication rate concerning wound healing and respiratory failure.
Videoscopic assistance
Although the anterior transmanubrial approach provides good exposure of the apical structures, an upper lobe lobectomy with hilar and mediastinal lymph node dissection may be difficult without a wide incision or an additional thoracotomy. Therefore, some authors propose to combine the anterior transmanubrial approach with video-assisted thoracic surgery (VATS) lobectomy (46-48). The VATS use may support the surgical procedure and improve surgical outcome (49) providing:
- Good overview of the superior sulcus area;
- Aid to confirm or change the surgical strategy intraoperatively;
- Aid to determine the appropriate chest wall resection level, avoiding the resection of extra ribs;
- Possibility to complete lobectomy and lymph node dissection avoiding a second incision;
- Less post-surgical pain due to less rib injury, less inter-costal nerve injury, less rib spreading, less length of skin incision and preservation of latissimus dorsi;
- Prompter recovery of pulmonary function;
- Reduction in opioid and analgesic consumption.
Limits to resectability
Pancoast tumor unresectability criteria remained unchanged for a long time (6,9,11,15,50,51), in recent years, however, some restrictions have been revised and absolute and relative contraindications to surgery have begun to change (Table 2).
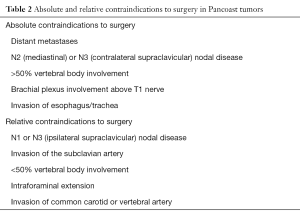
Full table
Concerning lymph node involvement, there is no agreement about N2 disease and surgical treatment. Some authors (25,35) excluded all N2 disease patients from surgical treatment. Others, like Kwong and colleagues (41), accepted well-defined mediastinal lymph nodes disease and subdued those patients to surgery only in case of chemotherapy response. No difference in survival between N2 and N0/N1 tumor involvement was found. In most papers (17,21,39,52-55), however, N+ disease is considered as a negative prognostic factor in terms of overall and disease-free survival. As previously stated, ipsilateral supraclavicular lymph node disease (N3 disease) is a peculiar finding and its biological behavior could be equalized to local nodes due to the anatomy of the region where Pancoast tumor occurs.
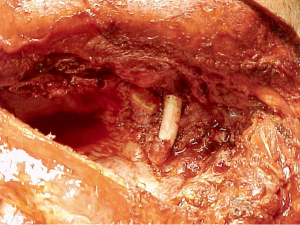
Vertebral body involvement greater than 50% was historically considered an absolute contraindication to surgery and a relative contraindication with an involvement lower than 50%. This limitation was caused by the lack of proper surgical techniques to achieve a radical resection and no multimodality treatment. At present, surgical procedures such as laminectomy, partial or hemivertebrectomy or complete resection of vertebral body can be performed in the context of a triple modality therapy, with high rates of complete surgical resection, good long-term survival, and acceptable morbidity and mortality (24,32,33,56,57) (Figure 7). The careful selection of patients and of specialized centers with interdisciplinary teams of thoracic and spine surgeons is indispensable.
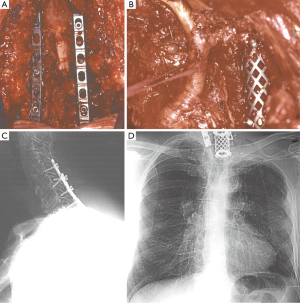
Vascular involvement, thanks to new surgical techniques and to the current multimodality therapy, is no longer a contraindication to resection. The subclavian vein can be resected with ligation of the vessel. The involved part of a subclavian artery can be resected and reconstructed (11,15,58) (Figure 8). Even the invasion of the common carotid and vertebral arteries are no longer considered absolute contraindications to surgery even if the resectability is strictly dependent on patient’s vessel state in term of atherosclerotic changes and patency of the contralateral vessels (11,51).
Complications
Heterogeneous mortality and morbidity rates are available in operative series of Pancoast tumors undergoing multimodality treatment. Operative mortality rate ranges from 0% to 6.9% and morbidity from 11% to 47% (25,29,35-43). In particular, the SWOG 9416 (35) and JCO 9806 (36) phase II trials showed operative mortality rates of 2.3% and 3% and morbidity rates of 52% and 14%, respectively.
Surgical complications
Thoracic complications
(I) Prolonged air leakage, (II) wound infection, and (III) wound dehiscence are frequent surgical complications. (IV) Bronchopleural fistula (BPF) is a challenging condition, mostly in patients receiving high dose radiotherapy. Development of sophisticated and precise (3D radiation) radiotherapy techniques and of surgical technical skills (avoiding skeletonization and devascularization of bronchial stumps and coverage with a vascularized flap) may contribute to minimize BPF incidence rate. (V) Air embolism into the subarachnoid space caused by spinal fluid leakage is a rare occurrence. (VI) Chest wall resection or pleural adhesion could results in hemothorax or (VII) bleeding. Several pulmonary complications are caused by incomplete lung expansion (VIII) lung atelectasis due to the accumulation of pulmonary secretions may evolve in (IX) pneumonia complicated or not by (X) empyema. Chest wall dyskinesia, phrenic nerve dissection, pain, air leak and ineffective drainage may also lead to incomplete lung expansion and evolve in (XI) respiratory failure associated or not with (XII) acute respiratory distress syndrome. A number of measures should be taken to achieve a complete lung expansion: adequate ventilation using mechanical support if needed, appropriate analgesia, targeted physiotherapy, secretions suction by bronchoscopy or through temporary tracheostomy. (XIII) Aesthetic or functional discomfort of the osteo-muscular compartment may occur, because of the superior girdle instability, using the transcervical anterior surgical approach. Chest wall deformities and scapula entrapment can occur using the postero-lateral approach.
Vascular complications
(I) Intraoperative tear or injury of the subclavian artery may lead to complex surgery to correct it; (II) subclavian vein thrombosis due to radiotherapy and surgical resection is a frequent condition; (III) ipsilateral forearm edema is associated with the division of the subclavian vein without revascularization, elevation of the arm to facilitate the venous drainage must be encouraged. Rarely (IV) chylothorax can occur caused by tumors invasion or unaware surgical damn.
Neurological complications
(I) Intrinsic hand muscle weakness (Klumpke-Déjérine syndrome) can be caused by C8 root division; while the division of T1 root affects the neural system less significantly. (II) Horner’s syndrome (miosis, ptosis, and enophthalmos) may be also caused by high dorsal root sympathectomy.
Chemo-radiotherapy complications
Chemo-radiotherapy could be associated with (I) pneumonitis; (II) peripheral neurologic dysfunctions; (III) esophagitis; (IV) infection and (V) symptomatic debilitation; (VI) hematologic toxicity and (VII) stomatitis are common chemotherapy complications.
Prognostic factors and relapses
Defining the prognostic factors of overall and disease-free survival for superior sulcus tumors is a challenging topic. Many authors have tried to identify them and a various number of tumor characteristics seems to be involved. After reviewing the body of literature, (I) complete resection and (II) complete pathological response to therapy turned out to be positive prognostic factors in various series (17,29,35-38,40,52,55,59-62); (III) T status has been recognized as a poor prognostic factor (11,17,21,29,36,52-55,59). In particular brachial plexus invasion (59), vertebral body (11,52,53) and great vessels involvements (11,53) are correlated to an higher risk of tumor recurrence. The presence of Horner syndrome at the diagnosis of Pancoast tumor is, equally, a poor prognostic factor (17,54) implying extensive neural involvement; (IV) positive lymph node status, as in NSCLC tumor, is also a significantly poor prognostic factor in Pancoast tumor (17,21,39,52-55). In the revised series of Alifano and colleagues (60), the presence of (V) associated major illness and the completeness of resection are the two most important factors affecting the long-term outcome. Sartori and coworkers (21) found the (VI) decrease of arm pain, after irradiation, as good prognostic factor.
The relapse pattern has varied through the years, according to treatment changes. In patients treated with bimodality therapy local recurrences were 70% of all recorded relapses (43,63). The percentage reduced to less than 30% of all recurrences in patients treated with trimodality therapy (35-36,40). The decrease of local recurrence rate seems to be due to improved surgical technique and appropriate staging. Currently, the pattern of failure has shifted to distant relapses (especially brain relapses) (40-41,44,50,64). This raises the question of prophylactic brain irradiation (PCI). There is no agreement on the possibility to perform PCI routinely on superior sulcus patients treated with trimodality therapy. Komaki and colleagues (65) reported 143 Pancoast tumor patients variously treated (radiotherapy alone, radiotherapy plus surgery and others). No statistical difference in brain metastasis rate were observed in PCI treated and non-PCI treated patients, although the PCI group was far less numerous than non-PCI group. Another study (39) analyzed 31 patients treated with chemotherapy and surgery for Pancoast tumor: of 13 patients subjected to PCI, one turned out to have brain metastasis, while 3 out of 13 non-PCI patients had the same relapse; the authors concluded that PCI seemed to offer adequate protection against brain metastases. In 2011 the RTOG 0214 trial (66) evaluated the efficacy of PCI in patients with advanced lung cancers (stage IIIA or IIIB) with a randomized study: they observed a decreased number of brain metastases in patients treated with PCI, without significant impact on survival (1-year follow-up). Taking into account the decreasing brain metastasis number trend, PCI can be considered in treatment protocols of further studies.
Conclusions and new perspectives
Multidisciplinary approach is of paramount importance in the management of Pancoast tumors. This rare disease necessitates specialist knowledge for each different treatment proposed. Surgical treatment of T4 disease requires the participation of a multidisciplinary surgical team. The collaboration between thoracic, spine, and vascular surgeons may be needed to achieve complete resection in vertebral and vascular tumor involvement that are no longer a contraindication to resection. Surgery for extensive involvement of the brachial plexus is the new hurdle to overcome in T4 disease management. Nodal involvement represents another field of discussion. Is N2 disease still a surgical contraindication after chemo-radiotherapy downstaging? Could ipsilateral supraclavicular N3 disease be considered as N1 disease taking into account the peculiar anatomy in which these tumors grow? With regards to radiotherapy treatment, the advent of intensity-modulated radiation therapy (IMRT), allowing the radiation dose to conform more precisely to the three-dimensional shape of the chest, may allow a radiotherapy dose escalation.
Acknowledgements
The authors thank Chiara Battistella and Michael McGlade for language editing assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chardack WM, Maccallum JD. Pancoast syndrome due to bronchiogenic carcinoma: successful surgical removal and postoperative irradiation; a case report. J Thorac Surg 1953;25:402-12. [PubMed]
- Hare ES. Tumor involving certain nerves. London Med Gazette 1838;1:16-8.
- Pancoast HK. Importance of careful roentgen ray investigations of apical chest tumors. JAMA 1924;83:1407-11. [Crossref]
- Pancoast HK. Superior pulmonary sulcus tumor. Tumor characterized by pain, Horner’s syndrome, destruction of bone and atrophy of hand muscles. JAMA 1932;99:1391-96. [Crossref]
- Tobías JW. Sindrome ápico-costo-vertebral doloroso por tumor apexiano: su valor diagnostico en el cáncer primitivo pulmonar. Rev Med Latino Am 1932;17:1522-56.
- Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J Thorac Dis 2013;5:S342-58. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6:S108-15. [PubMed]
- Kozower BD, Larner JM, Detterbeck FC, et al. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e369S-99S.
- Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast's syndrome. N Engl J Med 1997;337:1370-6. [Crossref] [PubMed]
- Shaw RR, Paulson DL, Kee JL. Treatment of Superior Sulcus Tumor by Irradiation Followed by Resection. Ann Surg 1961;154:29-40. [Crossref] [PubMed]
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [Crossref] [PubMed]
- Fraser RG, Pare JA. Diagnosis of diseases of the chest. 2nd ed. Philadelphia: WB Saunders, 1978.
- Paulson DL. Carcinomas in the superior pulmonary sulcus. J Thorac Cardiovasc Surg 1975;70:1095-104. [PubMed]
- Netter FH. The CIBA Collection of Medical Illustrations: Volume 7, Respiratory System. New York: CIBA Pharmaceutical Company, 1979.
- Rusch VW. Management of Pancoast tumours. Lancet Oncol 2006;7:997-1005. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest 2009;136:260-71. [Crossref] [PubMed]
- Ginsberg RJ, Martini N, Zaman M, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumor. Ann Thorac Surg 1994;57:1440-5. [Crossref] [PubMed]
- Walker JE. Superior sulcus pulmonary tumor (Pancoast syndrome). J Med Assoc Ga 1946;35:364. [PubMed]
- Herbut PA, Watson JS. Tumor of the thoracic inlet producing the Pancoast syndrome; a report of 17 cases and a review of the literature. Arch Pathol (Chic) 1946;42:88-103. [PubMed]
- Chardack WM, Maccallum JD. Pancoast tumor; five-year survival without recurrence or metastases following radical resection and postoperative irradiation. J Thorac Surg 1956;31:535-42. [PubMed]
- Sartori F, Rea F, Calabrò F, et al. Carcinoma of the superior pulmonary sulcus. Results of irradiation and radical resection. J Thorac Cardiovasc Surg 1992;104:679-83. [PubMed]
- Shahian DM, Neptune WB, Ellis FH Jr. Pancoast tumors: improved survival with preoperative and postoperative radiotherapy. Ann Thorac Surg 1987;43:32-8. [Crossref] [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [PubMed]
- Gandhi S, Walsh GL, Komaki R, et al. A multidisciplinary surgical approach to superior sulcus tumors with vertebral invasion. Ann Thorac Surg 1999;68:1778-84; discussion 1784-5.
- Wright CD, Menard MT, Wain JC, et al. Induction chemoradiation compared with induction radiation for lung cancer involving the superior sulcus. Ann Thorac Surg 2002;73:1541-4. [Crossref] [PubMed]
- Dartevelle PG. Herbert Sloan Lecture. Extended operations for the treatment of lung cancer. Ann Thorac Surg 1997;63:12-9. [PubMed]
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [Crossref] [PubMed]
- Bains MS, Ginsberg RJ, Jones WG 2nd, et al. The clamshell incision: an improved approach to bilateral pulmonary and mediastinal tumor. Ann Thorac Surg 1994;58:30-2; discussion 33. [Crossref] [PubMed]
- Marulli G, Battistella L, Perissinotto E, et al. Results of surgical resection after induction chemoradiation for Pancoast tumours †. Interact Cardiovasc Thorac Surg 2015;20:805-11; discussion 811-2. [Crossref] [PubMed]
- Bilsky MH, Vitaz TW, Boland PJ, et al. Surgical treatment of superior sulcus tumors with spinal and brachial plexus involvement. J Neurosurg 2002;97:301-9. [PubMed]
- York JE, Walsh GL, Lang FF, et al. Combined chest wall resection with vertebrectomy and spinal reconstruction for the treatment of Pancoast tumors. J Neurosurg 1999;91:74-80. [Crossref] [PubMed]
- Bolton WD, Rice DC, Goodyear A, et al. Superior sulcus tumors with vertebral body involvement: a multimodality approach. J Thorac Cardiovasc Surg 2009;137:1379-87. [Crossref] [PubMed]
- Collaud S, Waddell TK, Yasufuku K, et al. Long-term outcome after en bloc resection of non-small-cell lung cancer invading the pulmonary sulcus and spine. J Thorac Oncol 2013;8:1538-44. [Crossref] [PubMed]
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995;13:1880-92. [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 2008;26:644-9. [Crossref] [PubMed]
- Fischer S, Darling G, Pierre AF, et al. Induction chemoradiation therapy followed by surgical resection for non-small cell lung cancer (NSCLC) invading the thoracic inlet. Eur J Cardiothorac Surg 2008;33:1129-34. [Crossref] [PubMed]
- Li J, Dai CH, Shi SB, et al. Induction concurrent chemoradiotherapy compared with induction radiotherapy for superior sulcus non-small cell lung cancer: a retrospective study. Asia Pac J Clin Oncol 2010;6:57-65. [Crossref] [PubMed]
- Marra A, Eberhardt W, Pöttgen C, et al. Induction chemotherapy, concurrent chemoradiation and surgery for Pancoast tumour. Eur Respir J 2007;29:117-26. [Crossref] [PubMed]
- Pourel N, Santelmo N, Naafa N, et al. Concurrent cisplatin/etoposide plus 3D-conformal radiotherapy followed by surgery for stage IIB (superior sulcus T3N0)/III non-small cell lung cancer yields a high rate of pathological complete response. Eur J Cardiothorac Surg 2008;33:829-36. [Crossref] [PubMed]
- Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 2005;129:1250-7. [Crossref] [PubMed]
- Favaretto A, Pasello G, Loreggian L, et al. Preoperative concomitant chemo-radiotherapy in superior sulcus tumour: A mono-institutional experience. Lung Cancer 2010;68:228-33. [Crossref] [PubMed]
- Kappers I, van Sandick JW, Burgers JA, et al. Results of combined modality treatment in patients with non-small-cell lung cancer of the superior sulcus and the rationale for surgical resection. Eur J Cardiothorac Surg 2009;36:741-6. [Crossref] [PubMed]
- Pitz CC, de la Rivière AB, van Swieten HA, et al. Surgical treatment of Pancoast tumours. Eur J Cardiothorac Surg 2004;26:202-8. [Crossref] [PubMed]
- Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413-5. [PubMed]
- Truin W, Siebenga J, Belgers E, et al. The role of video-assisted thoracic surgery in the surgical treatment of superior sulcus tumors. Interact Cardiovasc Thorac Surg 2010;11:512-4. [Crossref] [PubMed]
- Linden PA. Video-assisted anterior approach to Pancoast tumors. J Thorac Cardiovasc Surg 2010;140:e38-9. [Crossref] [PubMed]
- Nakajima T, Watanabe A, Nakazawa J, et al. Transmanubrial approach with video-assisted thoracoscopic surgery for left superior sulcus tumour with dense adhesion after replacement of descending thoracic aorta. Interact Cardiovasc Thorac Surg 2012;14:906-8. [Crossref] [PubMed]
- Caronia FP, Fiorelli A, Ruffini E, et al. A comparative analysis of Pancoast tumour resection performed via video-assisted thoracic surgery versus standard open approaches. Interact Cardiovasc Thorac Surg 2014;19:426-35. [Crossref] [PubMed]
- Tamura M, Hoda MA, Klepetko W. Current treatment paradigms of superior sulcus tumours. Eur J Cardiothorac Surg 2009;36:747-53. [Crossref] [PubMed]
- Bruzzi JF, Komaki R, Walsh GL, et al. Imaging of non-small cell lung cancer of the superior sulcus: part 2: initial staging and assessment of resectability and therapeutic response. Radiographics 2008;28:561-72. [Crossref] [PubMed]
- Anderson TM, Moy PM, Holmes EC. Factors affecting survival in superior sulcus tumors. J Clin Oncol 1986;4:1598-603. [PubMed]
- Muscolino G, Valente M, Andreani S. Pancoast tumours: clinical assessment and long-term results of combined radiosurgical treatment. Thorax 1997;52:284-6. [Crossref] [PubMed]
- Attar S, Krasna MJ, Sonett JR, et al. Superior sulcus (Pancoast) tumor: experience with 105 patients. Ann Thorac Surg 1998;66:193-8. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472-83. [Crossref] [PubMed]
- Fadel E, Missenard G, Chapelier A, et al. En bloc resection of non-small cell lung cancer invading the thoracic inlet and intervertebral foramina. J Thorac Cardiovasc Surg 2002;123:676-85. [Crossref] [PubMed]
- Setzer M, Robinson LA, Vrionis FD. Management of locally advanced pancoast (superior sulcus) tumors with spine involvement. Cancer Control 2014;21:158-67. [PubMed]
- Dartevelle P, Macchiarini P. Surgical management of superior sulcus tumors. Oncologist 1999;4:398-407. [PubMed]
- Okubo K, Wada H, Fukuse T, et al. Treatment of Pancoast tumors. Combined irradiation and radical resection. Thorac Cardiovasc Surg 1995;43:284-6. [Crossref] [PubMed]
- Alifano M, D'Aiuto M, Magdeleinat P, et al. Surgical treatment of superior sulcus tumors: results and prognostic factors. Chest 2003;124:996-1003. [Crossref] [PubMed]
- Vos CG, Hartemink KJ, Blaauwgeers JL, et al. Trimodality therapy for superior sulcus tumours: evolution and evaluation of a treatment protocol. Eur J Surg Oncol 2013;39:197-203. [Crossref] [PubMed]
- Goldberg M, Gupta D, Sasson AR, et al. The surgical management of superior sulcus tumors: a retrospective review with long-term follow-up. Ann Thorac Surg 2005;79:1174-9. [Crossref] [PubMed]
- Wright CD, Moncure AC, Shepard JA, et al. Superior sulcus lung tumors. Results of combined treatment (irradiation and radical resection). J Thorac Cardiovasc Surg 1987;94:69-74. [PubMed]
- Peedell C, Dunning J, Bapusamy A. Is there a standard of care for the radical management of non-small cell lung cancer involving the apical chest wall (Pancoast tumours)? Clin Oncol (R Coll Radiol) 2010;22:334-46. [Crossref] [PubMed]
- Komaki R, Putnam JB Jr, Walsh G, et al. The management of superior sulcus tumors. Semin Surg Oncol 2000;18:152-64. [Crossref] [PubMed]
- Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol 2011;29:272-8. [Crossref] [PubMed]