Elderly selection on geriatric index assessment
Lung cancer has been the most common cancer-worldwide since 1985 and the median age at diagnosis is 71 years (1). The Surveillance, Epidemiology and End Results (SEER) data reported that 37.3% of the patients diagnosed with cancer were 75 years and older and 68.4% were 65 years and older, respectively (1). The age of 70 years is considered as the limit of senescence and clinical trials dedicated to elderly patients usually consider the enrollment of patients aged 70 years and above (2). In the 1990s, the management of elderly patients with advanced lung cancer relied on best supportive care (BSC) only. In 1999, vinorelbine was shown to improve survival and quality of life of elderly patients with metastatic non-small cell lung cancer (NSCLC) (3). Single agent chemotherapy thus became the standard of care of this population in 2004 American Society of Clinical Oncology (ASCO) guidelines (4). In 2011 a phase III clinical trial showed the superiority of monthly carboplatin—weekly paclitaxel doublet in comparison with gemcitabine or vinorelbine monotherapy (5). Median overall survival (OS) was indeed 10.3 months for doublet chemotherapy versus 6.2 months for mono-chemotherapy (hazard ratio 0.64; 95% CI, 0.52–0.78; P<0.0001) (5). From then on, guidelines recommended carboplatin-based doublet for first-line treatment of fit elderly patients and single-agent chemotherapy for less fit patients (6). However, there is no clear definition of fit elderly patients and this population is very heterogeneous. Furthermore, there is a high proportion of elderly patients with co-morbidities and co-medications (7) potentially interfering with functional status and chemotherapy pharmacokinetics. There is thus a need for a precise assessment of elderly patients in order to define fit and less fit patients and guide first-line lung cancer treatment.
Towards this purpose, the use of a comprehensive geriatric assessment (CGA) is recommended in several guidelines (6). CGA is a multidisciplinary and global approach dedicated to predict the mortality and morbidity of elderly patients diagnosed with cancer and to adapt the cancer treatment in this specific population (8). The international society of geriatric oncology (SIOG) recommends the use of several tools to assess the functional, psychological, cognitive and nutrition status, the risk of falls and the number and severity of co-morbidities (9). As this evaluation may be long and difficult in a busy routine practice, SIOG developed simplified screening tools to identify elderly patients in need of the complete CGA. If abnormal, a screening tool should be followed by a complete geriatric assessment. Several screening tools are validated including G8, Flemish version of the triage risk screening tool (TRST), groninger frailty indicator (GFI), vulnerable elders survey-13 (VES-13), senior adult oncology program 2 (SAOP 2) and abbreviated comprehensive geriatric assessment (aCGA). G8 was extensively studied and showed the most robust results with a high sensitivity and good specificity as a prognostic and predictive factor for outcomes. However, no specific screening tool is recommended or discouraged (10). The different evaluation instruments used for the screening or for CGA are summarized in Table 1.
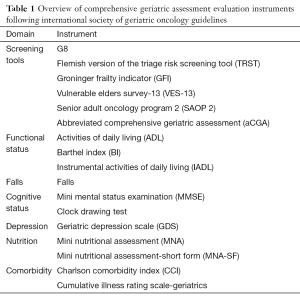
Full table
The implementation of the use of CGA in routine practice is not optimal. This may be due to the lack of recommendation and to the fact that there is no clear demonstration of the role of CGA on elderly cancer patients’ prognosis or clinical outcomes. In line with this, ASCO recently identified a critical need for specific guidelines for the treatment of older cancer patients (11).
The elderly selection on geriatric index assessment (ESOGIA) study recently published by Corre et al. in the Journal of Clinical Oncology compared treatment allocation based on age and performance status (PS) versus treatment allocation based on CGA (12). The main objective was to identify a survival difference between both arms. The primary objective was treatment failure free survival TTFS and secondary objectives were OS, progression-free survival (PFS), tolerability and quality of life. However, this study was negative. In the discussion section, the authors tempted to explain these negative results. To our opinion, several points have to be discussed.
First, the choice of endpoints and cut-offs may not be appropriate to show a difference between the CGA arm and the PS and age arm. TTFS was shown to be adapted to an older population of patients with advanced disease (13). But this composite endpoint taking into account not only treatment efficacy but also treatment toxicity is not a good endpoint for advanced NSCLC, as this patients with poor prognosis are more likely to die of lung cancer than of other causes. Moreover, the activities of daily living (ADL) scale and the instrumental activities of daily living (IADL) scale recommended to assess the functional status in CGA because of their association with survival (14) were not associated with TFFS in the ESOGIA study. The cut-offs chosen in this study followed previous recommendations established in a general population of elderly patients with cancer (15). However, there is no specific recommendation regarding the cut-offs for patients with advanced NSCLC, in relationship with their poor prognosis.
In addition, whereas SIOG guidelines recommend the assessment of nutrition status, no nutrition scale was used in the ESOGIA study. However, low body mass index has been shown to be associated with increased mortality, reduced tolerance to chemotherapy and shorter TFFS (16). Moreover, in this study, Charlson index was used to assess the number of comorbidities. This index was shown to be associated with prognosis and TFFS (8) but because of advanced NSCLC bad prognosis patients are more likely to die of lung cancer than any comorbidity. For this reason, the assessment of type and severity of comorbidity is more appropriate for advanced NSCLC than the number of comorbidities.
The lack of survival difference is also maybe due to the population of patients treated with BSC only: 23% in the CGA arm versus 0% in the PS and age group (12). The supplementary table A4 shows the number of patients in the PS and age group who would have received BSC only if treatment assignment had been performed with CGA: 27.3% in the group of patients treated with double therapy and 46% for patients treated with single therapy. CGA is thus an effective tool for the selection of patients who should not receive any chemotherapy, as shown in the vinorelbine versus BSC Italian study (3) and the IFCT 0501 study (5). However, some patients may have been undertreated as no patient with a PS of 2 or above was treated with chemotherapy doublet whereas phase III studies demonstrated that carboplatin-based chemotherapy was feasible for patients with a PS of 2 (17).
In the same way, CGA is a good tool to identify patients with a poor natural prognosis. As CGA-based treatment allocation is based on frailty, OS in the CGA arm for patients treated with BSC only or with single agent chemotherapy was thus shorter than in the PF and age arm (12). However, there is no data assessing whether BSC only is the appropriate option for these patients with poor natural prognosis.
Interestingly, the adverse event rate was lower in the CGA arm in comparison with the PS and age arm, even if older patients received chemotherapy in the CGA arm. The difference persisted but was not significant after the exclusion of patients treated with BSC only on the CGA arm. Fit elderly patients receiving carboplatin-paclitaxel doublet had the same OS in both arms but a better safety profile in the CGA arm. CGA is thus a helpful tool to select patients who can be treated safely and effectively with carboplatin-paclitaxel. In the IFCT 0501 trial, patients were not selected and 4.4% of patients in the carboplatin-paclitaxel arm died of treatment toxicity (5). CGA-based treatment allocation could have avoided this issue.
Finally, quality of life was improved in the CGA arm. Quality of life scores were similar at baseline and higher in the CGA arm for each subsequent assessment even for patients with BSC only.
In conclusion, we have to congratulate the investigators of the ESOGIA trial as even if the CGA-based treatment allocation does not improve survival of patients with advanced NSCLC it shows that CGA is an important tool for the selection of unfit patients who should receive BSC only and that CGA induces a reduction of adverse events and an improvement of quality of life.
The study focused on advanced NSCLC but it would have been interesting to assess the role of CGA in the early-stage setting. In this population of elderly patients with early-stage NSCLC, the benefit of surgery is well admitted and survival outcomes for patients aged 70 and above are similar to younger patients (18). However, regarding adjuvant chemotherapy, recommendations are still based on retrospective observational data (6). Cuffe et al. reported the data of 2,763 elderly patients undergoing surgery for early-stage NSCLC. Among patients treated with adjuvant chemotherapy, 70% received cisplatin- and 28% carboplatin-based regimens. Hospitalizations rates, requirements for dose adjustments were similar across age groups and survival improved with adjuvant chemotherapy in all subgroups except patients aged 80 years or above (19). These data highlight the need for a better selection of elderly patients able to receive cisplatin-based adjuvant chemotherapy.
Furthermore, to facilitate CGA application in routine care, there is a need for a better coordination between geriatricians performing the CGA and medical oncologists prescribing chemotherapy. The French National Cancer Institute demonstrated that only 8% of medical oncologists were trained in geriatric oncology (www.e-cancer.fr) whereas 48% of geriatricians were trained. Further studies are required to help the elaboration of recommendations regarding the practical organization of CGA for elderly patients with cancers.
In the near future, CGA also has to be evaluated for the assignment of new treatments such as targeted therapies or immunotherapies. Clinical outcomes and safety profile of these therapeutics indeed seem different for elderly patients than for younger patients with lung cancers. Subgroup analysis of the phase III trial comparing nivolumab with docetaxel for second line treatment of patients with adenocarcinoma of the lung (Checkmate 057) showed no survival benefit of nivolumab for patients aged 75 years or above, whereas nivolumab was superior to docetaxel for younger patients (20). These data suggest a different immune profile in the elderly population and a need for a better selection of patients who could potentially benefit from immunotherapy. Regarding targeted therapies, observational studies showed that elderly patients treated with erlotinib had similar survival and quality of life benefit than younger patients but experienced more adverse events (21). This again highlights the need for a good selection of elderly patients able to receive targeted therapies and CGA has to be investigated in this indication.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Editorial commissioned by Section Editor Jianrong Zhang, MD (Department of Thoracic Surgery, First Affiliated Hospital of Guangzhou Medical University, Guangzhou Institute of Respiratory Disease, Guangzhou, China)
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Balducci L. Geriatric oncology: challenges for the new century. Eur J Cancer 2000;36:1741-54. [Crossref] [PubMed]
- Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 1999;91:66-72. [Crossref] [PubMed]
- Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330-53. [Crossref] [PubMed]
- Quoix E, Zalcman G, Oster JP, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079-88. [Crossref] [PubMed]
- Pallis AG, Gridelli C, Wedding U, et al. Management of elderly patients with NSCLC; updated expert's opinion paper: EORTC Elderly Task Force, Lung Cancer Group and International Society for Geriatric Oncology. Ann Oncol 2014;25:1270-83. [Crossref] [PubMed]
- Chun SH, Lee JE, Park MH, et al. Gemcitabine Plus Platinum Combination Chemotherapy for Elderly Patients with Advanced Non-small Cell Lung Cancer: A Retrospective Analysis. Cancer Res Treat 2011;43:217-24. [Crossref] [PubMed]
- Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-31. [Crossref] [PubMed]
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241-52. [Crossref] [PubMed]
- Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol 2015;26:288-300. [Crossref] [PubMed]
- Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33:3826-33. [Crossref] [PubMed]
- Corre R, Greillier L, Le Caër H, et al. Use of a Comprehensive Geriatric Assessment for the Management of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: The Phase III Randomized ESOGIA-GFPC-GECP 08-02 Study. J Clin Oncol 2016;34:1476-83. [Crossref] [PubMed]
- Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer--Alliance for Clinical Trials in Oncology--International Society Of Geriatric Oncology position article. J Clin Oncol 2013;31:3711-8. [Crossref] [PubMed]
- Hennessy S, Kurichi JE, Pan Q, et al. Disability Stage is an Independent Risk Factor for Mortality in Medicare Beneficiaries Aged 65 Years and Older. PM R 2015;7:1215-25. [Crossref] [PubMed]
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Bozzetti F. Why the oncologist should consider the nutritional status of the elderly cancer patient. Nutrition 2015;31:590-3. [Crossref] [PubMed]
- Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013;31:2849-53. [Crossref] [PubMed]
- Yamamoto K, Padilla Alarcón J, Calvo Medina V, et al. Surgical results of stage I non-small cell lung cancer: comparison between elderly and younger patients. Eur J Cardiothorac Surg 2003;23:21-5. [Crossref] [PubMed]
- Cuffe S, Booth CM, Peng Y, et al. Adjuvant chemotherapy for non-small-cell lung cancer in the elderly: a population-based study in Ontario, Canada. J Clin Oncol 2012;30:1813-21. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Wheatley-Price P, Ding K, Seymour L, et al. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008;26:2350-7. [Crossref] [PubMed]


