
Effects of postoperative radiotherapy and docetaxel and PD-1 inhibitors on the survival and safety of glioblastoma patients: a systematic review and meta-analysis
Highlight box
Key findings
• Postoperative combination of RT and chemotherapy and PD-1 inhibitors had some advantages over docetaxel in terms of effectiveness, but more clinical studies are needed to confirm clinical effects.
What is known and what is new?
• The OS rate and safety of postoperative chemotherapy alone, or combined with RT, or PD-1 inhibitor therapy in patients with GBM is still unclear.
• Postoperative combination of RT and chemotherapy and PD-1 inhibitors had some advantages over docetaxel in terms of effectiveness, but more clinical studies are needed to confirm clinical effects.
What is the implication, and what should change now?
• However, because clinical research on PD-1 is still in the initial stages and there is limited understanding of its safety, extensive clinical studies are needed to evaluate its effectiveness and to better understand the intricacies of its application.
Introduction
Glioblastoma (GBM) is by far the predominantly malignant brain tumor in adulthood, 57% of all gliomas and 48% of all central nervous system (CNS) malignant tumors (1). It is characterized by high invasiveness, vascular richness, high recurrence rate, and a low cure rate. Despite the multi-modal standard treatment including surgery, radiotherapy (RT), and chemotherapy (2,3). Given its aggressive biological nature, GBM often relapses and has a poor prognosis. The median overall survival (OS) time is only 14.6 months, while the median progression-free survival (PFS) time is only 6.9 months (4). In addition, the significant deterioration of nervous system function and quality of life imposes a hefty burden on patients, families, and health care workers (5). The common therapies for newly diagnosed GBM contain maximum complete cytoreduction, postoperative RT, and temozolomide (TMZ) combined with targeted and supportive therapy. Despite extensive research, only tumor-treating fields (TTFs) therapy can enhance the prognosis of GBM patients (6). However, supporting the high cost of TTF in clinical practice in our country is not feasible. Therefore, extensive research is needed to investigate whether the current comprehensive treatment of GBM can be optimized to promote prognosis in GBM patients.
During postoperation, the patient receives RT and adjuvant TMZ (7-9). A meta-analysis of 6 studies revealed that postoperative RT had a significant survival benefit compared with no RT in glioma patients (10). Most of these studies used the older technique of three-dimensional conformal RT (3DCRT). With the development of RT technology, intensity modulated conformal RT (IMRT) has become the first choice for brain tumor RT because of its accuracy and safety. However, delineating tumor size strategies remains controversial. A multicenter study survey reflects the continuing variability of practice worldwide (11).
TMZ, the most important chemotherapeutic drug, exhibited promising good therapeutic potential in patients with recurrent GBM and thus was further studied by researchers (12,13). However, the controversy lies in the response rate, which states that half of the cases were tolerant to TMZ (14). Small molecular targeted drugs, such as erlotinib and gefitinib, do not achieve the same effect in the molecular subtypes and specific signaling pathways of gliomas as in other solid tumors (15,16).
Immune-checkpoint inhibitors (ICIs) have been found to be effective in the treatment of melanoma and non-small-cell-lung cancer (NSCLC) (17). Recently, cancerous cells have been recognized to trigger immune tolerance in the microenvironment by blocking co-suppressive signalings of cytotoxic T cells. Programmed cell death-1 (PD-1) inhibits T cell responses, promotes productions of T regulatory cells, and leads to T-cell tolerance (18). Tumor cells can also induce immunosuppression via expression of inhibitory cytokines, regulatory T lymphocytes, and myelogenous inhibitory cells to change the tumor microenvironment. In clinical trials abroad, antibodies against PD-1 have shown anti-tumor activities against chemotherapy-resistant NSCLC (19,20). Atzumab is selectively humanized-monoclone antibody against programmed cell death ligand 1 (PD-L1), which mainly acts on PD-1, thus preventing the interpalys between them. The binding inhibition leads to blockade of negative regulation of T lymphocytes and reverses tumor immune tolerance to establish a new immune response through the above mechanisms to achieve anti-tumor immunotherapy (21,22).
Despite multi-modal approaches augmented to treat GBM, the OS and 5-year survival rates are still unsatisfactory. Given the unsatisfactory prognosis of current GBM management, the urgent demand is for innovative therapeutic strategies. There remains a scarcity of information regarding the postoperative treatment of GBM and its effect on survival and safety using various combinational therapies, including chemotherapy alone, chemotherapy combined with RT, or PD-1 inhibitor therapies. Therefore, this meta-analysis aimed to investigate the postoperative treatment effects on efficacy and safety of GBM patients. These drugs and chemotherapy regimens containing docetaxel were analyzed through a systematic evaluation approach. We present the following article in accordance with the PRISMA reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2670/rc).
Methods
Inclusion and exclusion criteria
The inclusion criteria comprised that study subtypes: all subtypes of studies were searched of drug treatment in patients with GBM. Participants: (I) patients over 18 years of age; (II) patients with GBM confirmed by pathological and cytological diagnosis; and (III) patients with no autoimmune disease and ICIs. The patient’s sex, race, and nationality were not limited.
Intervention: the experimental cases accepted an anti-PD-1 drug and RT + chemotherapy, whereas the patients in the control group were treated with docetaxel alone. Outcome indicators: the therapeutic effect was measured using mean difference (MD) and 95% confidence interval (CI). Statistics were provided on the number of treatment-related and serious adverse reactions based on the grading standard of adverse reactions by WHO. The exclusion criteria were that studies that did not provide access to the full-text studies repeatedly published through multiple channels and studies in languages other than Chinese or English.
Outcome of interest
The primary outcomes of our interest included the OS and the PFS of the patient with GBM. The secondary outcomes include the measurement of adverse reactions.
Retrieval strategy
We searched online databases like PubMed, Embase, China National Knowledge Infrastructure (CNKI), Wanfang, and Chinese Science and Technology Periodicals (CQVIP) for clinical studies of PD-1 antibody therapy and docetaxel monotherapy or combination chemotherapy for GBM published up to May 2020. The main keywords used were GBM and PD-1.
Data extraction and quality evaluation
Two investigators independently extracted data according to a predesigned data extraction table, which was then cross-checked to ensure the accuracy of the data (23). The quality of the literature was evaluated using the Cochrane Risk of Bias 2.0. The individual studies were assessed for risk of bias with the Cochrane Collaboration tool. Finally, these items were categorized into low-quality literature [1–3] and high-quality literature [4–7].
Statistical analysis
RevMan5.4 software was derived from the Cochrane Collaboration Network Meta-analysis. The indicators studied were continuous variables, so the weighted mean difference (WMD) was chosen as the effect scale with a 95% CI. It was first χ2 test to determine heterogeneity, and if P>0.05 and I2<50%, it was considered homogeneous and the modified effect model was acquired for meta-analysis; if P<0.05 and I2≥50%, the combined effect was needed to determine homogeneity and a random-effects model was selected. During P<0.05, the original heterogeneities would not be chosen, descriptive analysis was applied.
Results
Literature screening
A total of 927 articles were all identified through the online searching. The literature was reviewed and evaluated. A total of 719 articles were excluded due to poor research quality, repetition, lack of information, or abnormal data. After reading completely, 20 articles (20/26 ariticles) not following the criteria were excluded, leaving 6 studies for inclusion in the study (Figure 1). The literatures included in the study included not only randomized controlled trial (RCT) (1,4,5) but also non-RCT (2,3,6).
Basic characteristics and quality evaluation of the included studies
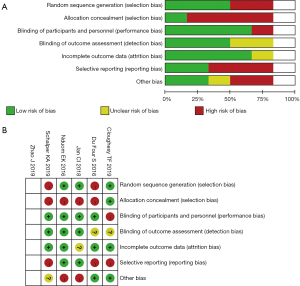
The basic features of the targeted researches are displayed as Table 1. Adverse reactions above grade III related to drug treatment are shown in Table 2. Six studies were graded using the Cochrane Risk of Bias 2.0 scale, with all 6 signifying high-quality literature(Table 3, Figure 2).
Table 1
| Serial number | First author | Number of patients | Publication year |
|---|---|---|---|
| 1 | Cloughesy TF | 35 | 2019 |
| 2 | Zhao J | 66 | 2019 |
| 3 | Nduom EK | 94 | 2016 |
| 4 | Schalper KA | 30 | 2019 |
| 5 | Du Four S | 18 | 2016 |
| 6 | Jan CI | 27 | 2018 |
Table 2
| First author | Adverse event | Incidence of grade 3 or higher AE (95% CI) |
Risk ratio (95% CI) |
|---|---|---|---|
| Cloughesy TF | Hepatitis | 0.43 (0.30–0.62) | 50.59 (32.01–80.31) |
| Zhao J | Lipase increased | 0.71 (0.51–0.98) | 42.01 (28.37–61.82) |
| Nduom EK | GGT increased | 0.47 (0.30–0.69) | 41.96 (25.27–66.53) |
| Schalper KA | Type 1 diabetes | 0.18 (0.10–0.30) | 41.86 (20.20–84.44) |
| Du Four S | Colitis | 0.47 (0.34–0.65) | 37.90 (25.49–56.49) |
| Jan CI | Increased transaminases | 0.13 (0.05–0.27) | 34.21 (12.46–84.56) |
AE, adverse event; CI, confidence interval; GGT, gamma-glutamyltransferase.
Table 3
| Include the literature | Year of publication | Random selection bias |
Allocation selection bias | Performance bias | Detection bias | Attrition bias |
Reporting bias |
Other bias |
|---|---|---|---|---|---|---|---|---|
| Cloughesy TF | 2019 | ① | ① | ③ | ② | ① | ③ | ① |
| Zhao J | 2019 | ① | ① | ① | ② | ③ | ① | ① |
| Nduom EK | 2016 | ① | ③ | ① | ① | ① | ① | ③ |
| Schalper KA | 2019 | ③ | ③ | ① | ① | ① | ③ | ② |
| Du Four S | 2016 | ③ | ③ | ① | ② | ① | ③ | ① |
| Jan CI | 2018 | ① | ③ | ① | ① | ② | ① | ③ |
, low risk; ②, unclear risk; ③, high risk.
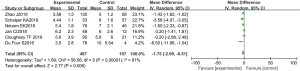
OS
Patients’ OS was recorded in all the studies, which included 270 individuals. Statistical heterogeneity was observed (P<0.00001; I2=91%). The study by Zhao involved two subgroups, 2 and 10 mg, and they were each calculated separately. The meta-analysis indicated PD-1 inhibitors effectiveness was higher than that of the control cohort (MD, −1.75; 95% CI: −2.99 to −0.51; P=0.006). The results suggested PD-1 efficacy alone treating advanced NSCLC surpass to docetaxel-based therapy (Figure 3).
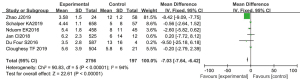
Treatment-related side effects
Significant differences were observed in the incidence of grade III–V serious adverse reactions such as granulocytopenia, anemia, and fatigue (MD, −7.03; 95% CI: −7.64 to −6.42; P<0.00001). After GBM treatment, the incidence of adverse reactions in the PD-1 antibody treatment group was lower. About 42.2% of patients had treatment-related side effects, particularly neutropenia and febrile neutropenia, suggesting that docetaxel usage may have raised patients’ treatment burden (Figure 4).
Publication bias
Funnel-plot findings were bilaterally symmetrical distribution, showing as unlikely published bias (Figure 5).
Discussion
Gliomas represent the deadliest form of brain tumor. Gliomas pose the greatest threat to human health among CNS malignancies due to their high malignancy, mortality, and impairment rates (23). According to data released by the National Cancer Center of China, about 70% of primary malignant brain tumors are gliomas, and the incidence is increasing yearly (24). GBM, also known as GBM multiforme, occurs mainly in the supratentorial region and accounts for 10–15% of intracranial tumors. This form of brain cancer is more common in people who are middle-aged and elderly, and the ratio of males to females is 2–3:1 (25). GBM often entails severe degeneration, hemorrhage, and necrosis due to tumor cells’ high degree of anaplasia and immaturity. In addition, there may be vascular reactions, including thrombosis. GBM is often characterized by invasive growth, frequently affecting multiple lobes (26). The tumor invades through the brain, often along the corpus callosum to the contralateral frontal lobe.
GBM is characterized by rapid growth and a short course of the disease. Generally, the onset of symptoms to medical treatment span 3 months. Individual cases with protracted illness histories are benign in the early stage and later transform into a malignancy (27). The tumor-induced severe edema results in increased intracranial pressure, with almost all cases involving headaches, vomiting, and optic nerve papilledema. The tumor invading the motor or sensory area will result in the occurrence of Hemiplegia, hemianesthesia, and motor or sensory aphasia. However, the incidence of epilepsy in patients with GBM was significantly lower than in astrocytoma and oligodendroglioma (27-29).
According to the WHO grading system, the degree of malignancy of a glioma increases gradually with the grade. GBM recurrence most often occurs in grades IV–V, the median survival time is no more than 14.6 months, and the 5-year survival rate is about 9.8%. Although the malignant degree of low-grade (grade I–II) gliomas is not as high as that of high-grade gliomas. The 5-year survival rate for low-grade (I–II) ranges from 30–70% (30). The main reasons for the poor therapeutic effect and clinical prognosis are the invasive growth of gliomas and blood-brain barrier (BBB) and the blood-tumor barrier, making it difficult for many therapeutic drugs to pass through (31).
In recent years, there have been numerous studies on PD-L1 and PD-1, which were first discovered in 1992 in interleukin-3 and mouse cell hybridoma derivatives (32). PD-1 is encoded by the programmed cell death 1 (PDCD1) gene, located on human chromosome 2, including 5 exons (32,33). As an immunosuppressive receptor, PD-1 can be expressed on different immune system cells, particularly cytotoxic T cells. The structure of PD-1 is similar to that of CD28, inducible costimulatory (ICOS) factor, and cytotoxic T lymphocyte-associated antigen 4, indicating PD-1 as a member of the CD28 superfamily. However, PD-1 is different from CD28 family members for its uniqueness, mainly attributable to the special sequence of the cytoplasmic tail of PD-1 (33). The PD-1 system’s receptors and ligands interact in a complicated way. PD-L1 is mainly expressed on the surface of different immune system cells, while PD-L2 is primarily expressed in dendritic cells of activated macrophages (34,35). In the tumor microenvironment, when PD-1 on the immune cells binds to PD-L1 on the tumor cells, the immune cells lose their anti-tumor effect, which is the basis of immune escape, re-exerting their anti-tumor effects (36).
Simultaneous RT and chemotherapy is a common treatments for GBM. However, this treatment model is changing with the rise of immunotherapy and the advent of PACIFIC research. The survival benefits of PD-1/PD-L1 inhibitor in GBM has promoted its application to GBM treatment (37). Some scholars have proposed that combining RT and immunotherapy can produce a synergistic effect (38). RT can activate the key immunized components, and enhanced immunogenic anti-tumor effects can overcome immunotherapy resistance (39). Immunotherapy combined with RT can increase antigen cross-presentation activity, promote tumor-infiltrating lymphocytes’ infiltration, and increase the expansion of effector T cells. Furthermore, RT has a synergistic effect on immunotherapy by decreasing the number of tumor cells, resulting in the upregulation of expressed PD-L1 in tumor and increasing exposure of new antigens, which can enhance the efficacy of immunotherapy.
Meanwhile, immunotherapy enhanced the sensitivity of RT to promote its efficacy (40,41). The extensively distant effect of RT immunogenicity, refers to the shrinking or disappearance of tumors at distant metastatic sites due to the release of circulating tumor antigen induced by radiation after the primary tumor treatment. An increase in immunogenicity of the tumor enhances the immune response to the tumor at distant metastatic sites (42). This has been recorded in mouse and human models, particularly melanoma and renal cell carcinoma. Although there is a lack of clinical evidence for GBM, these findings provide a good theoretical basis for the application of immunotherapy combined with RT in GBM.
PACIFIC study and a series of comparable investigations revealed that PD-1/PD-L1 monoclonal antibody has advantages in disease control, prolonging survival time, and improving patient quality of life in first-line at stage-III NSCLC suffers (43). Devaruzumab, an inhibitor of PD-1/PD-L1 binding (44,45), is already approved by the FDA US as first-line standard therapy after simultaneous RT and chemotherapy for patients with stage III NSCLC. The latest 4-year survival data of the PACIFIC study indicated median PFS of stage III NSCLC suffers treated by simultaneous RT and chemotherapy combined with devaruzumab was 17.2 months, while that of the placebo group was only 5.6 months (MD, 0.51), reducing disease advancement or mortality with 49%. The median OS was reported to be 47.5 months, 4-year PFS and OS rates were 35.3% and 49.6% respectively. Drug safety was found to be in an acceptable range (44). Overall, the results of this study have led to a breakthrough in stage III NSCLC treatment, changed the previous standard treatment model, and rewritten the treatment guidelines for stage III NSCLC patients (37). However, this treatment has not been widely promoted in China, attributed to high devaruzumab treatment cost, accessibility of drugs, physical quality and tolerance of Chinese people.
The PACIFIC studies have established the therapeutic status of immune-consolidation therapy in patients with GBM after simultaneous RT and chemotherapy (43-45). However, the selection of PD-1/PD-L1 inhibitors and the timing of RT and chemotherapy are still under exploration. The ECOG-ACRIN5181 study has been exploring whether OS in GMB will benefit from simultaneous RT and chemotherapy combined with devaruzumab. Similarly, the PACIFIC-2 study of simultaneous RT and chemotherapy with devaruzumab or placebo for GBM patients with stable disease or in remission, followed by consolidation therapy with devaruzumab or placebo, may help further to clarify the optimal timing for the administration of devaruzumab. Clinical studies on PD-L1 inhibitor atrazumab are also underway in patients with GBM. Atrazumab is a selective humanized monoclonal antibody against PD-L1 that prevents binding and interaction between PD-1 and PD-L1, blocking the negative regulation of T lymphocytes, and reversing the tolerance of tumor immunity. A new immune response is established through the above mechanisms to achieve the effect of anti-tumor immunotherapy (39-41).
DETERRED study was to assess the atrazumab application in patients with GBM. The study was divided into two parts, with safety as the primary goal. The first part was to continue adjuvant chemotherapy for 2 rounds after simultaneous RTs and chemotherapyies. The second part was to administer atrazumab to GBM suffers for 1 year after RT and chemotherapy. The adverse events > grade 3 in the first and second parts was 80%, and no grade 5 toxic reaction was related to immunotherapy (45). In the first part of the study, the median PFS and OS were 12.5 and 22.8 months. By second part, the median PFS 13.2 months, without the achieved median OS yet. In general, the safety of this study was within an acceptable range, and PFS and OS became longer compared to traditional simultaneous RT and chemotherapy, providing more evidence for the drug options for GBM.
A number of clinical studies on PD-1 inhibitors, including pablizumab, nivlumab, and other ICIs, are also underway. Nivolumab is a complete monoclonal immunoglobulin A (IgA) antibody whose main function is to bind to PD-1 to prevent it from binding to PD-L1. The NICOLAS study, a phase II single-arm study, included 79 patients with GBM. Patients received 1 cycle of induction chemotherapy followed by simultaneous RT and chemotherapy. Furthermore, the patients were administered two rounds of nivolumab 360 mg immunotherapy every 3 weeks, followed by 2 cycles of nivolumab 180 mg immunotherapy, and finally treated with nivolumab 480 mg immune-consolidation therapy for 1 year. The findings of the study revealed that the 1-year PFS rate was 57.3%, the median PFS time was 12.7 months, the 1-year OS rate was 63.7%, and the median OS time was 38.8 months.
Pablizumab is a T cell regulatory protein, which results in a negative regulatory signal through their interplays, producing a new anti-cancer response by inhibiting PD-1 or its ligand to enhance T cell activity (46). The LUN-47 study comprised 92 patients diagnosed histologically or pathologically with GBM who underwent immunoconsolidation therapy with pablizumab for 1 year within 28–56 days after the end of simultaneous RT and chemotherapy. The median PFS time of the patients in this study was 18.7 months, the 3-year PFS was 37.4%, the median OS was 35.8 months, and the 3-year OS rate was 48.5%. Subgroup analysis indicated no significant correlation between PFS and OS values and PD-L1 expression status. The nivolumab and pablizumab listing in China at a lower price than PD-L1 inhibitors may be a better choice for some patients, although more clinical data are needed to confirm its effectiveness.
A previous study has indicated that PD-L1 is expressed on cytoplasmic membrane of T lymphocytes, macrophages, and dendritic cells related to prognosis (44). However, there have been vast differences in the results obtained using different antibody cloning and detection methods to detect PD-L1. Varied antibodies have different affinities and epitopes, hence PD-L1 expression in GBM identified by different approaches may vary, leading to variations in the results of different studies. This study reviewed OS and PFS of patients with GBM treated with anti-PD-1 inhibitors and chemotherapy containing docetaxel in published studies. The findings revealed that patients treated with ICIs survived longer and responded better than those treated by docetaxel chemotherapies.
Furthermore, the incidence of grade III and above adverse reactions of nivolumab was lower than pembrolizumab or atezolizumab. The odds ratio (OR) of side effects associated with nivolumab was very low compared with studies with pembrolizumab and atezolizumab, which was consistent with Park et al. (47).
Conclusions
PD-1 antibody has some benefits in term of efficacy as compared with docetaxel and docetaxel-containing chemotherapy, and the incidence of adverse reactions above grade III is lower. However, because clinical research on PD-1 is still in the initial stages and there is limited understanding of its safety, extensive clinical studies are needed to evaluate its effectiveness and to better understand the intricacies of its application.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2670/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2670/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019;25:477-86. [Crossref] [PubMed]
- Zhao J, Chen AX, Gartrell RD, et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med 2019;25:462-9. [Crossref] [PubMed]
- Nduom EK, Wei J, Yaghi NK, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol 2016;18:195-205. [Crossref] [PubMed]
- Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 2019;25:470-6. [Crossref] [PubMed]
- Du Four S, Maenhout SK, Benteyn D, et al. Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion. Cancer Immunol Immunother 2016;65:727-40. [Crossref] [PubMed]
- Jan CI, Tsai WC, Harn HJ, et al. Predictors of Response to Autologous Dendritic Cell Therapy in Glioblastoma Multiforme. Front Immunol 2018;9:727. [Crossref] [PubMed]
- de Mello RA, Veloso AF, Esrom Catarina P, et al. Potential role of immunotherapy in advanced non-small-cell lung cancer. Onco Targets Ther 2017;10:21-30. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomized controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multi-centre randomized controlled trial. Lancet 2017;389:255-65. Erratum in: Lancet 2017;389:e5. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Zhao S, Jiang T, Zhou CC. Efficacy-predictive biomarkers of anti-PD-1/PD-L1 immunotherapy for non-small cell lung cancer. Tumor 2016;36:823-8.
- Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis 2016;3:198-210. [Crossref] [PubMed]
- Li J, Shangguan DG, Yuan SQ, et al. Research Progression on the Drug Therapy for Elderly Patients with Non-small Cell Lung Cancer. Anti-Tumor Pharmacy 2017;7:146-51.
- Fossella FV, DeVore R, Kerr RN, et al. Randomized trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 2000;18:2354-62. [Crossref] [PubMed]
- Armocida D, Pesce A, Di Giammarco F, et al. Histological, molecular, clinical and outcomes characteristics of Multiple Lesion Glioblastoma. A retrospective monocentric study and review of literature. Neurocirugia (Astur: Engl Ed) 2021;32:114-23.
- Lv L, Huang J, Xi H, et al. Efficacy and safety of dendritic cell vaccines for patients with glioblastoma: A meta-analysis of randomized controlled trials. Int Immunopharmacol 2020;83:106336. [Crossref] [PubMed]
- Wang T, Pham A, Yoo S, et al. Identifying Disparities in Care in Treating Glioblastoma: A Retrospective Cohort Study of Patients Treated at a Safety-net Versus Private Hospital Setting. World Neurosurg 2020;137:e213-20. [Crossref] [PubMed]
- Naumowicz M, Kusaczuk M, Kruszewski MA, et al. Corrigendum to "The modulating effect of lipid bilayer/p-coumaric acid interactions on electrical properties of model lipid membranes and human glioblastoma cells" [Bioorg. Chem. 92 (2019) 103242]. Bioorg Chem 2020;96:103607. Erratum for Bioorg Chem 2019;92:103242.
- Kamp MA, Rapp M, Cornelius JF, et al. Letter to the Editor Regarding “A Novel Wavelength-Specific Blue Light-Emitting Headlamp for 5-Aminolevulinic Acid Fluorescence-Guided Resection of Glioblastoma”. World Neurosurg 2020;133:436-7. [Crossref] [PubMed]
- Aguiar I, Ferreira E, Pontes R, et al. Dilemmas in primary spinal glioblastoma management during pregnancy. Rev Esp Anestesiol Reanim 2020;67:347-50. (Engl Ed). [Crossref] [PubMed]
- Shen SH, Woroniecka K, Barbour AB, et al. CAR T cells and checkpoint inhibition for the treatment of glioblastoma. Expert Opin Biol Ther 2020;20:579-91. [Crossref] [PubMed]
- Abbas MN, Kausar S, Cui H. Therapeutic potential of natural products in glioblastoma treatment: targeting key glioblastoma signaling pathways and epigenetic alterations. Clin Transl Oncol 2020;22:963-77. [Crossref] [PubMed]
- Greco C, Taresco V, Pearce AK, et al. Development of Pyrazolo[3,4-d]pyrimidine Kinase Inhibitors as Potential Clinical Candidates for Glioblastoma Multiforme. ACS Med Chem Lett 2020;11:657-63.
- Baehr A, Trog D, Oertel M, et al. Re-irradiation for recurrent glioblastoma multiforme: a critical comparison of different concepts. Strahlenther Onkol 2020;196:457-64. [Crossref] [PubMed]
- Yang X, Cao W, Wang X, et al. Down-regulation of 14-3-3zeta reduces proliferation and increases apoptosis in human glioblastoma. Cancer Gene Ther 2020;27:399-411. [Crossref] [PubMed]
- Yang K, Tang XJ, Xu FF, et al. PI3K/mTORC1/2 inhibitor PQR309 inhibits proliferation and induces apoptosis in human glioblastoma cells. Oncol Rep 2020;43:773-82. [Crossref] [PubMed]
- El Husseini K, Marguet F, Lamy A, et al. Major response to temozolomide as first-line treatment for newly-diagnosed DDR2-mutated glioblastoma: A case report. Rev Neurol (Paris) 2020;176:402-4. [Crossref] [PubMed]
- Kit Leng Lui S, Iyengar PV, Jaynes P, et al. Response to: USP26 regulates TGF-β signalling by deubiquitinating and stabilizing SMAD7; not applicable in glioblastoma. EMBO Rep 2020;21:e47269. [Crossref] [PubMed]
- Pucci C, De Pasquale D, Marino A, et al. Hybrid Magnetic Nanovectors Promote Selective Glioblastoma Cell Death through a Combined Effect of Lysosomal Membrane Permeabilization and Chemotherapy. ACS Appl Mater Interfaces 2020;12:29037-55. [Crossref] [PubMed]
- Park JE, Kim HS, Park SY, et al. Identification of Early Response to Anti-Angiogenic Therapy in Recurrent Glioblastoma: Amide Proton Transfer-weighted and Perfusion-weighted MRI compared with Diffusion-weighted MRI. Radiology 2020;295:397-406. [Crossref] [PubMed]
- Wang Z, Liu F, Liao W, et al. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch Biochem Biophys 2020;689:108412. [Crossref] [PubMed]
- Pudelko L, Rouhi P, Sanjiv K, et al. Glioblastoma and glioblastoma stem cells are dependent on functional MTH1. Oncotarget 2017;8:84671-84. [Crossref] [PubMed]
- Berberich A, Schmitt LM, Pusch S, et al. cMyc and ERK activity are associated with resistance to ALK inhibitory treatment in glioblastoma. J Neurooncol 2020;146:9-23. [Crossref] [PubMed]
- Londhe VY, Date V. Personalized neoantigen vaccines: a glimmer of hope for glioblastoma. Expert Rev Vaccines 2020;19:407-17. [Crossref] [PubMed]
- Timme CR, Degorre-Kerbaul C, McAbee JH, et al. The Olfactory Bulb Provides a Radioresistant Niche for Glioblastoma Cells. Int J Radiat Oncol Biol Phys 2020;107:194-201. [Crossref] [PubMed]
- Glynn AM, Rangaswamy G, O'Shea J, et al. Glioblastoma Multiforme in the over 70's: "To treat or not to treat with radiotherapy?". Cancer Med 2019;8:4669-77. [Crossref] [PubMed]
- Tabrizi S, Trippa L, Cagney D, et al. A Quantitative Framework for Modeling COVID-19 Risk During Adjuvant Therapy Using Published Randomized Trials of Glioblastoma in the Elderly. Neuro Oncol 2020;22:918-27. [Crossref] [PubMed]
- Guo X, Wang S, Wang Y, et al. Anti-PD-1 plus anti-VEGF therapy in multiple intracranial metastases of a hypermutated, IDH wild-type glioblastoma. Neuro Oncol 2021;23:699-701. [Crossref] [PubMed]
- Yao Z, Zhang X, Zhao F, et al. Ursodeoxycholic Acid Inhibits Glioblastoma Progression via Endoplasmic Reticulum Stress Related Apoptosis and Synergizes with the Proteasome Inhibitor Bortezomib. ACS Chem Neurosci 2020;11:1337-46. [Crossref] [PubMed]
- Molinari A, Iovenitti G, Mancini A, et al. AuNP Pyrazolo[3,4-d]pyrimidine Nanosystem in Combination with Radiotherapy against Glioblastoma. ACS Med Chem Lett 2020;11:664-70.
- Lakomy R, Kazda T, Selingerova I, et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front Oncol 2020;10:840. [Crossref] [PubMed]
- Balana C, Carrato C, Vaz MA. Reply to: “Extended adjuvant temozolomide in newly diagnosed glioblastoma: is more less?”. Neuro Oncol 2020;22:1889-90. [Crossref] [PubMed]
- Chen W, Liu D, Liu P, et al. Current evidence and challenges of systematic therapies for adult recurrent glioblastoma: Results from clinical trials. Chin J Cancer Res 2021;33:417-32. [Crossref] [PubMed]
- Mirzaei R, Yong W. STEM-04. PD-1 Independent of PD-L1 ligation promotes glioblastoma growth through the NFkB pathway. Neuro Oncol 2021;23:vi22. [Crossref] [PubMed]
- Park JE, Kim HS, Park SY, et al. Prediction of Core Signaling Pathway by Using Diffusion- and Perfusion-based MRI Radiomics and Next-generation Sequencing in Isocitrate Dehydrogenase Wild-type Glioblastoma. Radiology 2020;294:388-97. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)




