
Targeted activation of ERK1/2 reduces ischemia and reperfusion injury in hyperglycemic myocardium by improving mitochondrial function
Highlight box
Key findings
• Targeted activation of ERK1/2 can reduce diabetic IR damage.
What is known and what is new?
• ERK1/2 can alleviate myocardial ischemia-reperfusion injury, and diabetes can aggravate IR injury. Our study revealed that targeted activation of ERK1/2 improves mitochondrial function, reduces cardiomyocyte apoptosis, mitochondrial division and oxidative stress, thereby alleviating diabetic IR damage.
What is the implication, and what should change now?
• It can provide the basis for the precise treatment of coronary heart disease and guide the anti-inflammatory treatment of coronary heart disease.
Introduction
Coronary heart disease has become the leading cause of death worldwide (1). Diabetes not only significantly increases the risk of coronary heart disease but also increases the mortality rate of diabetics from coronary heart disease (2). A previous study has shown that for every 1% increase in glycosylated hemoglobin in diabetic patients, related cardiovascular events increase by 11–16% (3). Similarly, with fasting blood glucose >5.6 mmol/L, for every 1-mmol/L increase in fasting blood glucose, the risk of coronary heart disease increases by 12%, and the risk of death from related cardiovascular diseases increases by 13% (4). The all-cause mortality rate of cardiovascular disease in diabetic patients is 3 times higher than that of non-diabetic patients, and the cardiovascular mortality rate is 5 times higher (5).
Reperfusion therapy can induce fatal reperfusion injury while saving viable myocardium. Diabetes increases not only the difficulty and complexity of interventional procedures but also the occurrence of coronary artery dissection, perforation, no reflow or slow blood flow, bleeding, contrast-induced renal injury, and stent thrombosis, and restenosis, and significantly increases the risk of adverse cardiovascular events and heart failure in patients with myocardial infarction.
Extracellular signal-regulated kinase 1/2 (ERK1/2) is widely present in many organisms and is the key to signal transmission from cell surface receptors to the nucleus. A variety of stimulating factors, such as growth factors, cytokines, viruses, G protein-coupled receptors, oncogenes, ischemia, and hypoxia, can activate the ERK1/2 pathway and participate in cell proliferation, cell metabolism, cell morphology maintenance cytoskeleton construction, cell differentiation, cell canceration, cell apoptosis, and many other biological reactions (6-9). Studies have shown that ERK1/2 is an important protein kinase in the reperfusion injury salvage kinase pathway. The activation of the ERK1/2 can alleviate myocardial ischemia reperfusion (I/R) injury, reduce myocardial cell apoptosis, and protect the myocardium (10-15).
Many studies have shown that diabetes aggravates myocardial ischemia-reperfusion injury (16-20). However, the effect of hyperglycemia on the protective factor ERK1/2 and the mechanism of their interaction in aggravating myocardial I/R injury remain unclear. Thus, this study established an I/R model in vitro using cardiomyocytes cultured with high glucose (HG) to explore the mechanism of hyperglycemia myocardial I/R injury and the intervention effects. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5149/rc).
Methods
Vector design
The recombinant adeno-associated virus type9 (rAAV9) vector was purchased from Virovek (Hayward, CA, USA) and was produced by the recombinant baculovirus production system in SF9 cells as previously described (21). The rAAV9 vector uses a vector containing double-stranded deoxyribonucleic acid to package the enhanced green fluorescent protein (GFP) gene (dsAAV9-GFP) and the constitutive activation of the mitogen-activated protein kinase 1 gene (dsAAV9-CaMEK), which is driven by the human cytomegalovirus promoter.
Research objects
The rat H9C2 cardiomyocytes were purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences, Shanghai, China, and served as the research objects for this study.
Cardiomyocyte cultures
The H9C2 cardiomyocytes were cultured in Dulbecco’s Modified Eagle Medium (DMEM; 1 g/L D-Glucose, v/v, Gibco) containing 10% fetal bovine serum (FBS; v/v, Gibco, Australia) and 1% penicillin-streptomycin (PS, v/v, Hyclone, USA), and were then treated with HG I/R. LM22B-10 (10 umol/L, MedChemExpress, USA) was administered before the intervention. The cells were cultured in a 37 ℃ incubator with 5% carbon dioxide.
Transfection of cardiomyocytes
The 2 viruses of dsAAV9-eGFP or dsAAV9-CaMEK were diluted with DMEM according to the original concentration, and the multiplicity of infection was 1×107 vg/cell. The virus was transfected for 24 hours and cultured in normal medium for 5 days, and then photographed under a confocal microscope (21,22). Western blot was used to detect the effect of the virus transfection.
Experimental grouping and intervention
The experimental grouping interventions for this study was as follows:
- the Control (Con) group: cardiomyocytes were cultured in low-glucose (LG) complete medium until the cells were collected.
- the HG group: the cells were first routinely cultured and then cultured in HG complete medium for 24 hours until the intervention was over, after which, the cells were collected.
- the I/R group: the cells were first routinely cultured and then switched to LG ischemia medium (without any FBS). The oxygen was replaced with nitrogen, and the cells were aerated for 7 minutes. The cells were then cultured for 12 hours. The cells were then washed with phosphate-buffered saline (PBS) and treated with LG complete medium for 15 minutes of reperfusion. The cells were subsequently collected.
- the HG + I/R group: the cells were first routinely cultured and then cultured in the HG complete medium for 24 hours. At the end of the culture, the cells were washed with PBS and switched to HG ischemia medium. The nitrogen gas was replaced with oxygen, and the cells were aerated for 7 minutes. The cells were then incubated for 12 hours. Finally, the cells were washed with PBS and re-perfused in HG complete medium for 15 minutes before collection.
- the HG + I/R + LM22B-10 group: the cells were first routinely cultured and then cultured in HG complete medium. Next, 1 hour before ischemia treatment, HG complete medium + LM22B-10 was added to the plates and mixed thoroughly. After the intervention, the cells were switched to HG ischemia medium + LM22B-10 and mixed thoroughly. Next, the oxygen was replaced with nitrogen, and the cells were cultured for another 12 hours. After the culture was completed, the cells were washed with PBS, switched to complete HG medium + LM22B-10 in the dish, and mixed thoroughly. Finally, the cells underwent reperfusion for 15 minutes before cell collection.
- the HG + I/R + CaMEK group: the cells were first routinely cultured and transfected with the CaMEK gene. The cells were then washed with PBS and cultured in complete HG medium for 24 hours, and then underwent the I/R intervention. The cells were collected at the end of the intervention.
Determination of apoptosis by flow cytometry
The cells were divided into the following 6 groups: the Con group, HG group, IR group, HG + I/R group, HG + I/R + LM22B-10 group, and HG + I/R + CaMEK group. Cell apoptosis was assessed using the PE Annexin V Apoptosis Detection Kit I (BD Biosciences, USA). After the intervention for each group, the cell culture supernatant was collected, and the cells were washed with PBS and trypsinized (0.25%, Hyclone, USA). After digestion was terminated, the collected cell suspension and supernatant were centrifuged at 1,600 rpm for 5 minutes. The supernatant was discarded, and the cells were resuspended in 2 mL of cold PBS and counted. A total of 5×105 cells were collected and centrifuged in a low-temperature centrifuge at 4 ℃ with a rotation speed of 3,000 rpm and a centrifugal time of 5 minutes. The PBS was then discarded, and 100 µL of 1× binding buffer was used for each sample to resuspend the cells.
For the double staining group, 5 µL of PE Annexin V dye and 5 µL of 7-AAD dye were added to each tube to resuspend the cells (an unstained cell group and a single stained cell group were defined). Each tube received 400 µL of 1× binding buffer and was gently pipetted to mix the cardiomyocytes thoroughly, and the cells were filtered and loaded into the flow cytometer. The apoptosis of the cardiomyocytes was analyzed on the flow cytometer. The Q2 area represented the proportion of late cell apoptosis, the Q4 area represented the proportion of early cell apoptosis, the Q1 area represented necrotic cells, and the Q3 area represented living cells. Each group comprised 3 samples, and each experiment was repeated 3 times.
Determination of mitochondrial structure
The cells were inoculated into confocal dishes with 1×105 cells per dish. After the intervention, the culture medium was discarded, and the cells were rinsed with PBS. To each confocal dish, 1 mL of Mito-Tracker (Thermo Fisher Scientific, USA) working solution (500 nM) was added, mixed well, and incubated in a cell incubator for 20 minutes. The cells were washed 3 times with room temperature PBS (1 mL per wash), and confocal images were then taken.
Measurement of mitochondrial membrane potential
The cells were inoculated into confocal dishes with 1×105 cells per dish. After the intervention, the culture medium was discarded, and the cells were rinsed with PBS. To each confocal dish, 1 mL of the prepared JC-1 (Thermo Fisher Scientific, USA) working solution (10 µg/mL) was added, mixed well, and incubated in the cell incubator for 15 minutes. The staining solution was then discarded. The cells were washed 3 times with room temperature PBS (1 mL per wash), and confocal images were then taken.
Determination of oxidative stress
The cells were inoculated into confocal dishes with 1×105 cells per dish. After the intervention, the culture medium was discarded, and the cells were rinsed with PBS. To each confocal dish, 1 mL of Mito-SOX (Thermo Fisher Scientific, USA). Working solution (5 µM) was added, mixed well, and incubated in the cell incubator for 10 minutes. The staining solution was then discarded. The cells were washed 3 times with preheated PBS (1 mL per wash), and confocal images were then taken.
Determination of cellular immunofluorescence
After the completion of each intervention, the cells were washed with cold PBS twice, fixed in cold 4% tissue cell fixative solution (Beijing, Solarbio, China) for 15 minutes at room temperature, washed again with cold PBS, and incubated with 0.25% Triton-x100 (Beijing, Solarbio, China) for 10 minutes. The cells were then washed with PBS and blocked in 1% bovine serum albumin (BSA, Beijing, Solarbio, China) for 30 minutes at room temperature. The cells were incubated with primary antibodies (rabbit anti-dynamin-related protein 1 Drp1,1:100, Cell Signaling Technology, USA) and mouse anti-translocase of outer mitochondrial membrane 20 (TOMM20, 1:100, Abcam, Britain) at 4 ℃ overnight. Next, the cells were washed with PBS and incubated with the secondary antibody (goat anti-mouse 1:400, goat anti-rabbit 1:400, Abcam, Britain) for 1 hour in the dark. After washing with PBS, the nucleus was stained with DAPI (4’, 6-diaminidine-2-phenylindole) solution for 10 minutes. After washing with PBS again, images were taken under a confocal microscope.
Western blot analysis
After the completion of the intervention, the cell culture plates were immediately placed on ice, and the cells were rinsed with PBS buffer, lysed with RIPA lysis buffer (Beijing, Solarbio, China), and collected in a 1.5-mL EP (eppendorf) tube, and then lysed on ice for 10 minutes. The samples were centrifuged at 4 ℃ and 13,000 rpm for 15 minutes, and the Protein Quantification Kit (BCA Assay, Thermo Fisher Scientific, USA) method was used for the protein quantification. Next, 10–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Beijing, Solarbio, China) was used to separate proteins of different molecular weights, followed by blocking in 5% fat-free milk. The primary antibodies against p-ERK1/2, ERK1/2, MEK, total Drp1 (T-Drp1), Drp1s616, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Bcl-2, and Bax were used; the dilution ratios of the antibodies were all 1:1,000. All the primary antibodies were provided by Cell Signaling Technology, USA. Bcl-2 and Bax were provided by Abcam, Britain. Next, the samples were incubated with the antibodies overnight at 4 ℃. After washing, the membrane was incubated with the secondary antibody (goat anti- rabbit IgG, Cell Signaling Technology, USA) at 1:5,000 for 2 hours, and washed again. The membrane then underwent electrochemiluminescence (ECL, Millipore, USA) color development, and imaging (Bio-Rad Laboratories, USA).
Quality control
The following steps were taken to ensure quality control:
- Strict aseptic procedures were followed, and each group of experiments was repeated at least 3 times;
- The key steps were performed by professional and technical personnel;
- Professionals applied the double-blind method when measuring the experimental results and truthfully recorded the experimental process and results in detail.
Statistical analysis
The statistical analysis of the data was conducted using the SPSS 23.0 statistical software package (SPSS, Chicago, IL). The measurement data are expressed as the mean ± standard error of the mean (SEM). A 1-way analysis of variance was used to compare means between multiple groups, and statistically significant differences are represented by *P<0.05, **P<0.01, &P<0.05, &&P<0.01, #P<0.05, ##P<0.01.
Results
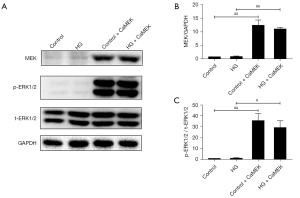
The CaMEK gene activated the MEK, and ERK1/2 protein expression levels in cardiomyocytes
The transfection of the CaMEK gene significantly upregulated the MEK protein expression level (P<0.01), and thus increased the phosphorylated-protein ERK1/2 expression level in the control group and HG group (P<0.05) (Figure 1).
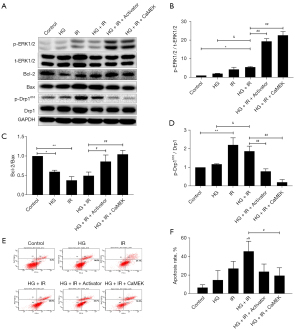
The CaMEK gene activated the expression level of phosphorylated-protein ERK1/2 and reduced myocardial apoptosis in HG + IR injury
The cells were divided into the following 6 groups: the Con group, HG group, I/R group, HG + I/R group, HG + I/R + LM22B-10 group, and HG + I/R + CaMEK group. The cells were treated with different intervention methods. After each intervention, the total protein of the cardiomyocytes was extracted, and the expression of the ERK1/2 protein and the expression of the apoptosis-related proteins in the cardiomyocytes were detected by Western blotting. Cardiomyocyte apoptosis was detected by flow cytometry. The results are shown in Figure 2. The phosphorylation level of the ERK1/2 protein increased after HG or I/R stimulation. Compared to that of the HG group, the phosphorylation level of ERK1/2 protein did not increase further after HG + I/R; however, the expression of the proapoptotic protein Bax increased, the expression of the anti-apoptotic protein Bcl-2 decreased, the ratio of Bcl-2/Bax decreased in the cells stimulated by HG + I/R, and the ratio of myocardial cell apoptosis further increased with HG + I/R stimulation (P<0.05). However, after the administration of LM22B-10 or the CaMEK gene transfection, the expression level of phosphorylated ERK1/2 protein increased (P<0.01), the expression of the proapoptotic protein Bax decreased, the expression of the anti-apoptotic protein Bcl-2 increased, the ratio of Bcl-2/Bax was increased (P<0.05), and the proportion of apoptosis was reduced in the HG + I/R group (P<0.05), indicating that both treatments can activate ERK1/2 and reduce myocardial cell apoptosis.
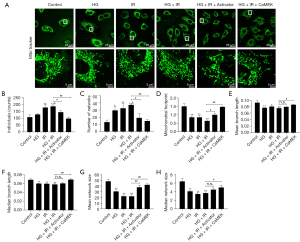
The CaMEK gene reduced mitochondrial fission in HG + I/R injury
A fluorescence probe (Mito-Tracker) was used to observe the structure of the mitochondria in HG + I/R injury. The results are shown in Figure 3. Compared to the results of the Con group, the HG or I/R stimulation increased mitochondrial fission, increased the number of punctate mitochondria, decreased the average branch length (HG group P<0.01, and I/R group P<0.05), and decreased the median network structure size, average network structure size, and median branch length (P<0.01). Compared to those in the HG group, the HG + I/R stimulation further increased the number of punctate mitochondria and further increased the mitochondrial fission (P<0.05). After LM22B-10 administration, the number of punctate mitochondria in the HG + I/R group decreased (P<0.05), the average network structure size increased (P<0.01), and the average branch length of the mitochondria, the median branch length, and the median size of the network structure increased. After transfection of the CaMEK gene, the number of punctate mitochondria in the HG + I/R group was further reduced, the average network structure size increased, the median branch length increased (P<0.01), and the median network structure size and the average branch length increased (P<0.05).
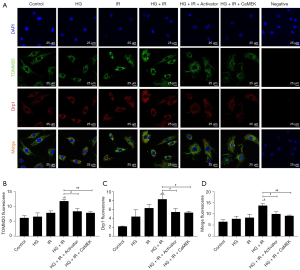
Western blotting and immunofluorescence were used to detect the expression of mitochondrial fission protein Drp1 and Drp1s616 phosphorylation, and the expression of the mitochondrial marker protein TOMM20. The results are shown in Figure 2. The expression of phosphorylated Drp1s616 in the HG group, I/R group, and HG + I/R group was significantly higher than that in the Con group. Compared to that in the HG group, the expression of phosphorylated Drp1s616 in the HG + I/R group was further increased (P<0.05).
LM22B-10 administration or CaMEK gene transfection reduced the phosphorylation of mitochondrial fission protein Drp1s616 in the HG + I/R group (P<0.01). The immunofluorescence results are shown in Figure 4. Compared to those in the Con group, the expression levels of Drp1 and TOMM20 in the HG group and the I/R group were increased. Compared to the HG group, the HG + I/R group had significantly higher expression levels of Drp1 and TOMM20 (P<0.05), but after LM22B-10 administration, the expression of TOMM20 was reduced (P<0.05), and the expression of Drp1 was significantly reduced (P<0.05). The transfection of the CaMEK gene reduced the expression levels of the Drp1 and TOMM20 proteins (P<0.01), thereby reducing mitochondrial fission and improving mitochondrial function. Together, these experimental results showed that the administration of LM22B-10 or transfection of the CaMEK gene inhibited Drp1s616 phosphorylation, reduced mitochondrial fission, and improved mitochondrial function.
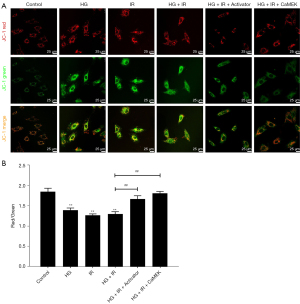
The CaMEK gene increased myocardial cell membrane potential in HG + I/R injury
We used JC-1 to detect the mitochondrial membrane potential, and the results are shown in Figure 5. Compared to that of the Con group, the red-green fluorescence ratio of the HG group and I/R group was significantly reduced (P<0.01). Compared to that of the HG group, the mitochondrial membrane potential further decreased with HG + I/R stimulation. After administering LM22B-10 or transfecting the CaMEK gene, the ratio of red to green fluorescence increased significantly (P<0.01). The results suggest that both treatments effectively improved the reduced mitochondrial membrane potential caused by HG + I/R and stabilized the function of mitochondria.
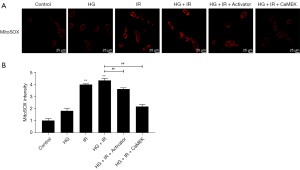
The CaMEK gene reduced the level of oxidative stress in HG + I/R injury
Mito-SOX was used to detect the level of reactive oxygen species (ROS) in the mitochondria. The results are shown in Figure 6. Compared to that in the Con group, the ROS level of the HG group increased, and the ROS levels of the I/R group and the HG + I/R group increased significantly (P<0.01). However, after the administration of LM22B-10 or CaMEK gene transfection, the level of ROS was significantly reduced (P<0.01), and the level of oxidative stress was reduced.
Discussion
In this study, we established a HG myocardial IR model in vitro to simulate hyperglycemia myocardial I/R injury and found that under HG or IR conditions, phosphorylated ERK1/2 protein expression was activated. However, with HG + I/R stimulation, the expression of the phosphorylated ERK1/2 protein did not increase further. Administering LM22B-10 or transfecting the CaMEK gene activated phosphorylated ERK1/2 protein expression, reduced mitochondrial fission, increased myocardial cell membrane potential, improved mitochondrial function, and reduced oxidative stress, myocardial cell apoptosis, and HG + I/R damage.
As an extracellular signal-regulated kinase (ERK) protein, ERK1/2 is involved in the occurrence and development of diabetes and cardiovascular diseases. Its main role is related to the regulation of cell apoptosis, hypertrophy, proliferation, and differentiation. However, the role of the ERK1/2 in myocardial damage caused by diabetes remains controversial. In myocardial ischemia-reperfusion injury, hyperglycemia can increase its susceptibility. The main pathological outcome of diabetes comes from the effect of hyperglycemia on the microvascular and cardiovascular system, and patients with diabetes are at increased risk of cardiovascular disease (23). A previous study has shown that ERK1/2, as an important protein kinase, is involved in myocardial ischemia-reperfusion injury and the reduction of myocardial apoptosis (10). Many studies have shown that the upregulation of ERK1/2 has a protective effect on the diabetic heart, and the activation of ERK1/2 improves I/R and drug-induced myocardial injury (10,24-26). Conversely, some studies have shown that under diabetic conditions, activation of the ERK1/2 is an important cause of oxidative stress, inflammation, cardiac remodeling, and apoptosis in the diabetic heart, and the inhibition of ERK1/2 reduces HG-stimulated cardiomyocyte apoptosis (11). In a db/db type 2 diabetic mice and a streptozotocin-induced type 1 diabetes model, mito-TEMPO (a mitochondrial targeted antioxidant) was found to effectively prevent the activation of ERK1/2 in cardiomyocytes under HG conditions and thereby protect the myocardium, indicating that the inhibition of ERK1/2 can prevent the death of cardiomyocytes caused by diabetes (27).
In the present study, we found that under HG or myocardial I/R conditions, the ERK1/2 was activated, which in turn led to cell apoptosis. However, treatment with both HG and myocardial I/R did not significantly activate the ERK1/2, but cell apoptosis was further increased, indicating that failure to activate the ERK1/2 under hyperglycemia myocardial I/R is an important reason for the death of a large number of cardiomyocytes and the aggravation of myocardial I/R injury. To verify the role of the ERK1/2 in hyperglycemia myocardial I/R injury, we established a hyperglycemia myocardial I/R injury model and found that LM22B-10 administration and CaMEK gene transfection activated the ERK1/2, improved mitochondrial function and oxidative stress, and reduced cell apoptosis (Figures 1,2).
Mitochondria are dynamic organelles that play an important role in cell survival and death. Mitochondria consist of a network of membranous tubules, and the morphology of mitochondria varies in different cell types. Morphological changes in mitochondria are mainly caused by the fission and fusion of mitochondrial tubules, which lead to the elongation of the mitochondria and the fragmentation of interconnected mitochondrial networks into discontinuous mitochondria (28,29). The fine balance between intracellular mitochondrial fusion and fission may be disturbed by many factors, including oxidative stress (30) and ischemia (31).
Mitochondria are highly dynamic, and are involved in many processes, including apoptosis, cell cycle, intracellular Ca2+(Calcium) homeostasis, Cell differentiation, Oxidative stress (28,29,32-35). In cardiomyocytes, mitochondria have a high density and their main role is to produce ATP, which is accomplished through respiratory metabolism and oxidative phosphorylation. When the function of mitochondria changes, the energy production of cardiomyocytes will also change, resulting in changes such as apoptosis of cardiomyocytes (36). Mitochondrial dynamics related protein 1(Drp1) can mediate mitochondrial division, and high glucose can induce mitochondrial dysfunction through it (37). HG has been shown to induce mitochondrial fragmentation and promote mitochondrial fission (38,39). Mitochondrial fission is the upstream cause of increased ROS due to hyperglycemic injury, and the inhibition of mitochondrial fragmentation inhibits the hyperpolarization of mitochondria caused by HG, normalize ROS levels, and reduce apoptosis, indicating that the occurrence of mitochondrial fragmentation in HG cultures occurs earlier than the increase in ROS (38,40).
HG has been shown to induce ERK1/2 activation, thereby activating mitochondrial fission (41). In this study, following HG + I/R stimulation, the flow cytometry results showed a significant increase in apoptosis (Figure 2). The Mito-Tracker results showed that the number of punctate mitochondria increased, and the average branch length of mitochondria, median branch length of mitochondria, average network structure size, and median network structure size were shortened, and the immunofluorescence results showed Drp1/TOMM20 colocalization, suggesting mitochondrial fission (Figures 3,4). JC-1 detection of the mitochondrial membrane potential showed that HG + I/R reduced the ratio of red to green fluorescence, suggesting a decrease in mitochondrial membrane potential (Figure 5). The Mito-SOX results showed that the simultaneous intervention with HG and myocardial I/R increased ROS levels, suggesting an increase in oxidative stress (Figure 6). LM22B-10 administration or CaMEK gene transfection activated phosphorylated ERK1/2 protein expression, reduced the phosphorylation of mitochondrial fission protein Drp1s616, increased mitochondrial membrane potential, reduced oxidative stress, and thereby reduced HG + I/R injury.
Mitogen-activated protein kinase (MAPK) is an important signal transfection cascade and has a central role in cell growth, differentiation, apoptosis, and transformation (42). MAPK is composed of a series of sequentially acting kinases that ultimately lead to dual phosphorylation and the activation of the terminal kinase p38 (p38 mitogen-activated protein kinase), c-Jun N-terminal kinases (JNKs), and ERKs (43). The main upstream activators of ERK1/2 are 2 MPAKS, MEK1, and MEK2, which directly phosphorylate the double sites on ERK kinase (Thr-Glu-Tyr) (43). Recent studies have shown that introducing the CaMEK gene into cells efficiently activates the ERK1/2 pathway, reduces mitochondrial damage, and inhibits cardiomyocyte apoptosis, and that CaMEK transgenic mice can effectively resist myocardial IR injury, with a significant reduction in the area of myocardial infarction.
Researchers have found that after being introduced into the mice and cells, the constitutively active CaMEK gene (a mutated MEK1 gene in which Ser218 and Ser222 are replaced with Glu) efficiently activates the ERK1/2 and regulates cell proliferation, differentiation, and apoptosis (7). Lips et al. (44) found that CaMEK transgenic mice effectively recovered from myocardial IR injury, and the area of myocardial infarction after IR was significantly reduced. In our study, we found that following an intervention with both HG and I/R, the transfection of the CaMEK gene significantly activated ERK1/2, inhibited mitochondrial fission, increased mitochondrial membrane potential, improved oxidative stress, and reduced cell apoptosis. Thus, it is likely that the targeted transfection of CaMEK genes will become an effective means for preventing hyperglycemia I/R injury, which is of great significance for the further treatment and prognosis of hyperglycemic myocardial ischemia-reperfusion injury, and provides ideas for the prevention and treatment of diabetic myocardial ischemia-reperfusion injury. This study was confined to cells and future studies should include in vivo experiments (such as electron microscope) for further verification and further study on other factors or drugs affecting myocardial ischemia-reperfusion injury in diabetes mellitus, as well as cell necrosis.
Conclusions
In HG + I/R injury, administering LM22B-10 or transfecting the CaMEK gene can activate ERK1/2 protein phosphorylation, reduce mitochondrial fission, increase mitochondrial membrane potential, improve mitochondrial function, and reduce oxidative stress and cardiomyocyte apoptosis, thereby reducing HG + I/R injury.
Acknowledgments
The authors would like to thank all the individuals and participants who supported this project.
Funding: This study was supported by the Xinjiang Uygur Autonomous Region Efficient Scientific Research Program Project Contract (grant No. XJEDU20211015), the National Natural Science Foundation of China (grant No. 81700315), and the State Key Lab of Pathogenesis, Prevention, and Treatment of High Incidence Diseases in Central Asia Fund (grant Nos. SKL- HIDCA-2019-43, SKL- HIDCA-2020-2).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5149/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5149/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5149/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Narvaez Linares N F, Poitras M, Burkauskas J, et al. Neuropsychological Sequelae of Coronary Heart Disease in Women: A Systematic Review. Neurosci Biobehav Rev 2021;127:837-851. [Crossref] [PubMed]
- Franco OH, Steyerberg EW, Hu FB, et al. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007;167:1145-51. [Crossref] [PubMed]
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-12. [Crossref] [PubMed]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22. [Crossref] [PubMed]
- Beckman JA, Paneni F, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 2013;34:2444-52. [Crossref] [PubMed]
- Wainstein E, Seger R. The dynamic subcellular localization of ERK: mechanisms of translocation and role in various organelles. Curr Opin Cell Biol 2016;39:15-20. [Crossref] [PubMed]
- Zou J, Lei T, Guo P, et al. Mechanisms shaping the role of ERK1/2 in cellular senescence Mol Med Rep 2019;19:759-70. (Review). [PubMed]
- Cho YY. RSK2 and its binding partners in cell proliferation, transformation and cancer development. Arch Pharm Res 2017;40:291-303. [Crossref] [PubMed]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J 2010;277:2-21. [Crossref] [PubMed]
- Li DY, Tao L, Liu H, et al. Role of ERK1/2 in the anti-apoptotic and cardioprotective effects of nitric oxide after myocardial ischemia and reperfusion. Apoptosis 2006;11:923-30. [Crossref] [PubMed]
- Xu Z, Sun J, Tong Q, et al. The Role of ERK1/2 in the Development of Diabetic Cardiomyopathy. Int J Mol Sci 2016;17:2001. [Crossref] [PubMed]
- McCubrey JA, Steelman LS, Chappell WH, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 2012;3:1068-111. [Crossref] [PubMed]
- Zhan L, Li D, Liang D, et al. Activation of Akt/FoxO and inactivation of MEK/ERK pathways contribute to induction of neuroprotection against transient global cerebral ischemia by delayed hypoxic postconditioning in adult rats. Neuropharmacology 2012;63:873-82. [Crossref] [PubMed]
- Pignataro G, Esposito E, Sirabella R, et al. nNOS and p-ERK involvement in the neuroprotection exerted by remote postconditioning in rats subjected to transient middle cerebral artery occlusion. Neurobiol Dis 2013;54:105-14. [Crossref] [PubMed]
- Yu B, Song B. Notch 1 signalling inhibits cardiomyocyte apoptosis in ischaemic postconditioning. Heart Lung Circ 2014;23:152-8. [Crossref] [PubMed]
- Li J, Zhao Y, Zhou N, et al. Dexmedetomidine Attenuates Myocardial Ischemia-Reperfusion Injury in Diabetes Mellitus by Inhibiting Endoplasmic Reticulum Stress. J Diabetes Res 2019;2019:7869318. [Crossref] [PubMed]
- Li W, Li W, Leng Y, et al. Ferroptosis Is Involved in Diabetes Myocardial Ischemia/Reperfusion Injury Through Endoplasmic Reticulum Stress. DNA Cell Biol 2020;39:210-25. [Crossref] [PubMed]
- Xie D, Zhao J, Guo R, et al. Sevoflurane Pre-conditioning Ameliorates Diabetic Myocardial Ischemia/Reperfusion Injury Via Differential Regulation of p38 and ERK. Sci Rep 2020;10:23. [Crossref] [PubMed]
- Cao C, Liu HM, Li W, et al. Role of adiponectin in diabetes myocardial ischemia-reperfusion injury and ischemic postconditioning. Acta Cir Bras 2020;35:e202000107. [Crossref] [PubMed]
- Sun M, Wang R, Xia R, et al. Amelioration of myocardial ischemia/reperfusion injury in diabetes: A narrative review of the mechanisms and clinical applications of dexmedetomidine. Front Pharmacol 2022;13:949754. [Crossref] [PubMed]
- Chen H. Exploiting the Intron-splicing Mechanism of Insect Cells to Produce Viral Vectors Harboring Toxic Genes for Suicide Gene Therapy. Mol Ther Nucleic Acids 2012;1:e57. [Crossref] [PubMed]
- Chen BD, He CH, Chen XC, et al. Targeting transgene to the heart and liver with AAV9 by different promoters. Clin Exp Pharmacol Physiol 2015;42:1108-17. [Crossref] [PubMed]
- Penna C, Andreadou I, Aragno M, et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia-reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br J Pharmacol 2020;177:5312-35. [Crossref] [PubMed]
- Tai W, Shi E, Yan L, et al. Diabetes abolishes the cardioprotection induced by sevoflurane postconditioning in the rat heart in vivo: roles of glycogen synthase kinase-3β and its upstream pathways. J Surg Res 2012;178:96-104. [Crossref] [PubMed]
- Gross ER, Hsu AK, Gross GJ. Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes 2007;56:127-36. [Crossref] [PubMed]
- Chen HT, Yang CX, Li H, et al. Cardioprotection of sevoflurane postconditioning by activating extracellular signal-regulated kinase 1/2 in isolated rat hearts. Acta Pharmacol Sin 2008;29:931-41. [Crossref] [PubMed]
- Ni R, Cao T, Xiong S, et al. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med 2016;90:12-23. [Crossref] [PubMed]
- Dimmer KS, Scorrano L. (De)constructing mitochondria: what for? Physiology (Bethesda) 2006;21:233-41. [Crossref] [PubMed]
- Hausenloy DJ, Scorrano L. Targeting cell death. Clin Pharmacol Ther 2007;82:370-3. [Crossref] [PubMed]
- Skulachev VP, Bakeeva LE, Chernyak BV, et al. Thread-grain transition of mitochondrial reticulum as a step of mitoptosis and apoptosis. Mol Cell Biochem 2004;256-257:341-58. [Crossref] [PubMed]
- Brady NR, Hamacher-Brady A, Gottlieb RA. Proapoptotic BCL-2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL-1 cells and GFP biosensors. Biochim Biophys Acta 2006;1757:667-78. [Crossref] [PubMed]
- Shah M, Chacko LA, Joseph JP, et al. Mitochondrial dynamics, positioning and function mediated by cytoskeletal interactions. Cell Mol Life Sci 2021;78:3969-86. [Crossref] [PubMed]
- Xin Y, Zhang X, Li J, et al. New Insights Into the Role of Mitochondria Quality Control in Ischemic Heart Disease. Front Cardiovasc Med 2021;8:774619. [Crossref] [PubMed]
- Bai Y, Wu J, Yang Z, et al. Mitochondrial quality control in cardiac ischemia/reperfusion injury: new insights into mechanisms and implications. Cell Biol Toxicol 2022; Epub ahead of print. [Crossref] [PubMed]
- Huang J, Li R, Wang C. The Role of Mitochondrial Quality Control in Cardiac Ischemia/Reperfusion Injury. Oxid Med Cell Longev 2021;2021:5543452. [Crossref] [PubMed]
- Paradies G, Paradies V, Ruggiero FM, et al. Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury: implications for pharmacological cardioprotection. Am J Physiol Heart Circ Physiol 2018;315:H1341-H1352. [Crossref] [PubMed]
- Zhang MY, Zhu L, Bao X, et al. Inhibition of Drp1 ameliorates diabetic retinopathy by regulating mitochondrial homeostasis. Exp Eye Res 2022;220:109095. [Crossref] [PubMed]
- Yu T, Sheu SS, Robotham JL, et al. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 2008;79:341-51. [Crossref] [PubMed]
- Kobayashi S, Zhao F, Zhang Z, et al. Mitochondrial Fission and Mitophagy Coordinately Restrict High Glucose Toxicity in Cardiomyocytes. Front Physiol 2020;11:604069. [Crossref] [PubMed]
- Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A 2006;103:2653-8. [Crossref] [PubMed]
- Yu T, Jhun BS, Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal 2011;14:425-37. [Crossref] [PubMed]
- Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol 1999;11:211-8. [Crossref] [PubMed]
- Widmann C, Gibson S, Jarpe MB, et al. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 1999;79:143-80. [Crossref] [PubMed]
- Lips DJ, Bueno OF, Wilkins BJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 2004;109:1938-41. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


