
BAG3 promotes autophagy and suppresses NLRP3 inflammasome activation in Parkinson’s disease
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disease. The foremost pathological manifestation of PD is the progressive loss of dopaminergic neurons in the substantia nigra, which leads to motor and non-motor manifestations (1). According to reports, many factors may relate to the pathogenesis of PD, including environmental, genetic, and aging factors (2), while microscopically, neuroinflammation, autophagy disorder, and oxidative stress can lead to the death of dopaminergic neurons (3-5).
Bcl2-associated athanogenes (BAGs) belong to the BAG family which contains proteins (BAG1–7) (6). Of the seven BAG proteins, BAG3 is the only one involved in key cellular fate decisions (7), and acts as an important helper for selective degradation of aggregate proteins through autophagy (8). BAG3 possesses three important functional domains, namely WW, BAG, and PXXP (9). In our previous study, we reported BAG3 could bind with heat-shock protein (HSP)70 to form the BAG3-HSP70 complex through its BAG domain and promote autophagy to degrade α-synuclein (a key pathogenic factor in PD) (10). In addition, the BAG3-HSP70 complex can regulate transcription factor nuclear factor (NF)-κB, and activation of NF-κB can also upregulate expression of BAG3 and trigger autophagy (11,12). These studies suggest that BAG3 may be involved in the inflammatory process, but the relationship between BAG3 and neuroinflammation is not clear.
Microglia in the brain of PD patients are in a pathological state of long-term activation, and induced neuroinflammation is an important factor in this (13). NOD-like receptor family, and the pyrin domain-containing 3 (NLRP3) inflammasome is the first inflammasome found and most studied in PD (14). And the expression level of NLRP3 inflammasome in activated microglia in PD models increased (15). It is suggested that the increase of NLRP3 inflammasome is a key part of neuroinflammation induced by microglia. We have reported that pramipexole suppresses neuroinflammation in PD models through Drd3-dependent autophagy (16), which prompted us to explore whether BAG3 could modulate PD-related neuroinflammation through autophagy.
In the present study, we explored the role of BAG3 in PD inflammation and demonstrated that overexpression of BAG3 could significantly reduce neuroinflammation and dopaminergic neuronal loss in PD mice induced by lipopolysaccharide (LPS) injection. In addition, we demonstrated that an autophagy suppressed LPS-induced inflammatory environment could be restored by overexpression of BAG3, which may be the mechanism by which it reduces neuroinflammation. More importantly, we found the overexpression of BAG3 inhibited activation of the NLRP3 inflammasome and caspase-1 in microglia induced by LPS. In summary, we found that BAG3 promotes autophagy and suppresses NLRP3 inflammasome formation in PD. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5159/rc).
Methods
Drugs and antibodies
A protocol was prepared before the study without registration. LPS (L2880) was purchased from Sigma (St. Louis, MO, USA). The primary antibodies were showed in Table 1.
Table 1
| Antigen | Vendor | Catalog number |
|---|---|---|
| BAG3 | Proteintech | 10599-1-AP |
| LC3 | NOVUS | NB100-2220 |
| p62 | Sigma | P0068 |
| TH | Abcam | ab112 |
| DAT | Millipore | MAB369 |
| Iba1 | Wako | 019-19741 |
| NLRP3 | AdipoGen Life Sciences | AG-20B-0014-C100 |
| Beclin1 | Cell Signaling Technology | 3738 |
| Atg5 | Cell Signaling Technology | 1299s |
| β-actin | Sigma | A3584 |
| GAPDH | Abclone | AC002 |
| Caspase-1 | Cell Signaling Technology | 24232 |
| Cleaved caspase-1 | Cell Signaling Technology | Asp296, 67314 |
BAG3, Bcl2-associated athanogene 3; LC3, light chain 3; TH, tyrosine hydroxylase; DAT, dopamine transporter; NLRP3, NOD-like receptor family, and the pyrin domain-containing 3.
The BAG3-short interfering RNAs (siRNAs) (forward: 5'-AAAUGUAACAACAUUAAAGCC-3', reverse: 5'-AAAUGUAACAACAUUAAAGCC-3') were purchased from GenePharma (Shanghai, China).
Cell culture
Mouse microglial BV2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) and with 10% fetal bovine serum (FBS) (10099141C; Gibco, Waltham, MA, USA) and 1% penicillin/streptomycin in an incubator at 37 ℃ and 5% CO2. BV2 cells were transfected with BAG3-siRNA by Lipofectamine RNAiMAX (13778-150; Thermo Fisher, Waltham, MA, USA) to knock down BAG3. BV2 cells were treated with or without LPS (1,000 ng/mL) for 24 h.
SH-SY5Y cells were cultured in DMEM/F12 (DF12) medium with 10% FBS and 1% penicillin/streptomycin in an incubator at 37 ℃ and 5% CO2.
Animal experiments
A total of 36 male specific pathogen-free (SPF) C57BL/6J mice aged 6–8 weeks were purchased from GemPharmatech Co., Ltd. (Nanjing, China). Experiments were performed under a project license (No. 202206A0353) granted by the Ethics Committee of Soochow University, in compliance with the institutional guidelines for the care and use of animals. All mice were maintained under a 12-h light/12-h dark cycle with food and water ad libitum. The experimental design and execution were performed by different individuals. To verify the role of BAG3 in neuroinflammation, we chose to overexpress BAG3 in the brain of mice and then use LPS to induce neuroinflammation. For the LPS-induced PD model, mice were randomly divided into four groups, and all subsequent operations were performed randomly to minimize potential confounders. The groups were labeled as (I) Con-adeno-associated virus (AAV) group; (II) BAG3-AAV group; (III) LPS + Con-AAV group; and (IV) LPS + BAG3-AAV group. To induce overexpression of BAG3 in vivo, mice in groups II and IV were stereotactically injected with AAV9-GV577-BAG3 (1×1013 vg/mL; Shanghai Genechem Co., Ltd., Shanghai, China) into the bilateral striatum according to the coordinates of anteroposterior (AP) +0.9 mm, mediolateral (ML) ±1.5 mm, and dorsoventral (DV) −3.5 mm (relative to the bregma) at a volume of 1 µL/side. Mice in groups I and III received an equal volume of negative AAV. We used previously reported methods to build a LPS-induced PD model (16). Three weeks later, mice in groups III and IV were treated with LPS (5 µg dissolved in 1 µL saline) in the bilateral striatum (1 µL/site, respectively) by stereotaxic injection according to the coordinates at (I) AP +1.18 mm, ML ±1.5 mm, DV −3.5 mm; (II) AP −0.34 mm, ML ±2.5 mm, and DV −3.2 mm from bregma. Mice in groups I and II were stereotactically injected with an equal volume of saline. After surgery, mice were kept on a warm pad until recovery, then placed back in the cage. All animals were included in the analysis.
Western blotting
Striatal tissues and cells were lysed in RIPA buffer (P0013B; Beyotime, Nantong, China) containing protease and phosphatase inhibitors (MCE, Shanghai, China). After centrifugation at 12,000 g for 20 min at 4 ℃, the supernatant was harvested and the protein concentration was measured by a BCA Protein Assay Kit (23225; Thermo Scientific, Waltham, MA, USA). Samples were separated on 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes then blocked with 5% nonfat milk powder in tris-buffered saline with Tween 20 (TBST) buffer at room temperature for 1 h. Samples were then incubated overnight at 4°C with primary antibodies, and the next day, membranes were incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibody (1:8,000; Jackson Laboratories, Bar Harbor, ME, USA) at room temperature for 1 h. Protein density was measured using an electrochemiluminescence (ECL) kit (P10300; NCM Biotech, Shanghai, China) and quantified by Image J software (National Institutes of Health, USA).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with Trizol reagent (15596018; Invitrogen, Carlsbad, CA, USA) and transcribed by the complementary DNA (cDNA) synthesis kit (K1622; Thermo Scientific, Vilnius, Lithuania). qPCR was performed with SYBR Green (A25778; ABI, Vilnius, Lithuania) by the 7500 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA), with 18S serving as an internal control gene. The primer sequences (Genscript, Nanjing, China) were showed in Table 2.
Table 2
| Name | Sequence |
|---|---|
| IL-1β-F | TGGAAAAGCGGTTTGTCTT |
| IL-1β-R | TACCAGTTGGGGAACTCTGC |
| TNF-α-F | CATCTTCTCAAAATTCGAGTGACAA |
| TNF-α-R | TGGGAGTAGACAAGGTACAACCC |
| IL-6-F | GAGGATACCACTCCCAACAGACC |
| IL-6-R | AAGTGCATCATCGTTGTTCATACA |
| 18S-F | TCAACACGGGAAACCTCAC |
| 18S-R | CGCTCCACCAACTAAGAAC |
IL, interleukin; TNF, tumor necrosis factor.
Microglial culture supernatant (MCS) transfer model
To test the neurotoxic effects of activated microglia, BV1 µg/mL LPS for 24 h to become activated, and a medium of BV2 cells was collected and mixed with fresh DF12 medium at a ratio of 1:1 (v/v). SH-SY5Y cells were cultured normally for 24 h and incubated with this conditioned medium for 12 h to induce cell apoptosis and death. The cells were then washed three times with phosphate-buffered saline (PBS) and collected.
Flow cytometry
Apoptosis of SH-SY5Y cells cultured in the MCS transfer model was quantified using the Annexin V-FITC Apoptosis Detection Kit (C1062L; Beyotime). The cells were pelleted and resuspended in 195 µL Annexin V-FITC binding solution, then 5 µL Annexin V-FITC and 10 µL propidium iodide staining solution were added, followed by incubation at room temperature (shielded from light) for 15 min. Apoptosis of the SH-SY5Y cells was assayed by flow cytometry (BD FACSVerse, BD Biosciences, San Jose, CA, USA).
Immunohistochemistry
After LPS or saline injection for 21 days, mice were anesthetized with 2% isoflurane and perfused with saline followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The mice brains were removed and post-fixed in 4% paraformaldehyde overnight, then dehydrated through a series of 10–30% sucrose solution at 4 ℃. Striatum and midbrain coronal sections were cut at 20 µm thickness on a cryostat (Leica, Wetzlar, Germany) then washed in 0.1 M PBS and blocked in 2% bovine serum albumin containing 0.1% Triton X-100 for 1 h at room temperature. Primary antibodies against tyrosine hydroxylase (TH) (1:1,000), dopamine transporter (DAT) (1:1,000) and Iba1 (1:1,000) were incubated at 4 ℃ overnight. Sections were incubated with HRP-conjugated secondary antibody for 1 h and stained using a 3,3'-diaminobenzidine (DAB) kit (GK500705; Gene Tech, Shanghai, China). Images were obtained under bright field by microscopy (MSX11; MSHOT, Guangzhou, China). For TH-positive cells in substantia nigra pars compacta (SNpc) analysis, one out of every neighboring six sections per mouse (120 µm interval) with the same anatomical structure was selected.
Statistical analysis
All data are presented as mean ± standard error of mean (SEM). All statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). One-way analysis of variance followed by Tukey’s post hoc analysis was performed to analyze differences in treatments, and P<0.05 was considered significant: *P<0.05, **P<0.01, ***P<0.001; and ns, not significant.
Results
Knockdown of BAG3 promotes inflammation in BV2 cells
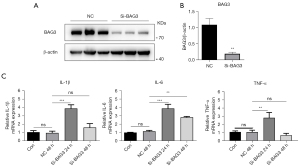
While in our previous study, we demonstrated that BAG3 regulates autophagy in PD (10), the relationship between BAG3 and neuroinflammation induced by BV2 microglia has not been studied. We hypothesized that BAG3 is related to the activation of BV2 microglia, and to test this we transfected BAG3 siRNA into the BV2 cell line to knock down expression of BAG3 (Figure 1A). The results showed that compared with the normal control (NC) group, the BAG3 in the si-BAG3 group decreased by 82% (Figure 1B), and inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 in BV2 cells increased significantly 24 h after transfection (Figure 1C).
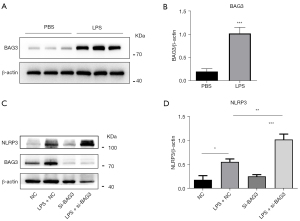
Knockdown of BAG3 promotes activation of NLRP3 inflammasome in LPS-induced BV2 cells
To verify the role of BAG3 in microglia-induced neuroinflammation, we used LPS to activate microglia. Western blotting showed expression of BAG3 in activated microglia was significantly increased, indicating it was involved in the activation of BV2 microglia (Figure 2A,2B). The NLRP3 inflammasome is an intracellular protein complex that regulates caspase-1 activity and IL-1β production. We have previously observed a significant increase in IL-1β in BV2 cells with reduced BAG3, so we speculated whether BAG3 was related to activation of the NLRP3 inflammasome during activation of BV2 cells. To test this hypothesis, we transfected BAG3 siRNA into BV2 cells for 24 h and treated them with LPS for 24 h. Expression of the NLRP3 inflammasome was detected by Western blotting (Figure 2C), and the results showed it increased significantly after knockdown of BAG3 (Figure 2D), confirming our hypothesis that BAG3 was involved in activation of the NLRP3 inflammasome in microglia induced by LPS.
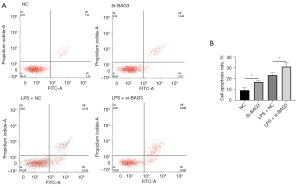
Knockdown of BAG3 aggravates neuronal death and apoptosis following microglial activation in MCS transfer model
Inflammatory cytokines released by activated microglia are an important pathway of neuronal death in PD patients and PD model animals, and this process can be simulated in vitro by the MCS transfer model (17). To verify whether the neuroinflammation involved in BAG3 promoted neuronal death, we transfected BAG3 siRNA into BV2 cells for 24 h, and treated them with LPS for 24 h. Medium of BV2 cells was collected and mixed with fresh DF12 medium at a ratio of 1:1 (v/v), and SH-SY5Y cells were incubated with this conditioned medium for 12 h before the apoptotic level of neurons was evaluated (Figure 3A). The cells were transfected with BAG3 siRNA, and the percentage of apoptotic dopaminergic cells was significantly higher than that of the controls (Figure 3B).
Overexpression of BAG3 reduces LPS-induced TH loss and microglia activation
Neuroinflammation is an important pathological process of PD and plays an indispensable role in disease progression. To explore the effect of BAG3 on neuroinflammation and dopaminergic neuronal damage in a PD model, we used stereotactic surgery to inject BAG3-AAV into the striatum of C57BL/6 mice to overexpress BAG3. After 21 days, LPS was injected into the striatum to establish a PD model related to neuroinflammation. According to our previous study, the expression level of inflammation was highest at 3 days after LPS injection and the loss of dopaminergic neurons in substantia nigra was higher after 21 days (16). Therefore, we detected expression of inflammatory cytokines after 3 days after LPS injection, and expression of TH and Iba1 in the substantia nigra and striatum after 21 days (Figure 4A). Compared with the Con-AAV group, the number of TH-positive neurons in the SNpc of the LPS + Con-AAV group decreased by 38.2% after 21 days, suggesting LPS-induced neuroinflammation significantly reduces the number of dopaminergic neurons. The loss of TH-positive neurons in the LPS + BAG3-AAV group was significantly lower than in the LPS + Con-AAV group (Figure 4B,4C), meanwhile, the expression of DAT in the LPS + BAG3-AAV group was significantly lower than in the LPS + Con-AAV group (Figure 4D,4E), suggesting the overexpression of BAG3 can protect TH neurons in an inflammatory environment.
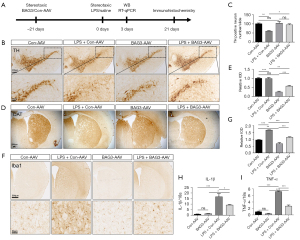
We then explored whether BAG3 affected the neuroinflammatory responses in a mouse model, and immunohistochemistry revealed LPS injection had a marked effect on the proliferation and activation of microglia in the striatum (Figure 4F,4G). Specifically, compared with the Con-AAV group, the number of microglia (Iba1+) increased, and the microglial cell bodies increased in size in the LPS group. Compared with the LPS + Con-AAV group, the number of microglia in the LPS + BAG3-AAV group decreased significantly, and the morphological changes in microglia were also less. RT-qPCR showed the inflammatory cytokines IL-1β and TNF-α were significantly enhanced in the LPS + Con-AAV group, while overexpression of BAG3 reduced IL-1β and TNF-α in the striatum, compared to the LPS + Con-AAV group (Figure 4H,4I).
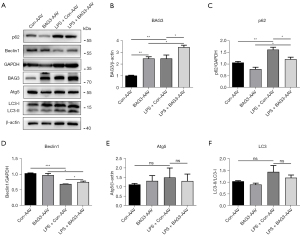
Overexpression of BAG3 enhances autophagy in an inflammatory environment
Autophagy disorders are found in the brains of both PD patients and PD models, indicating this process plays an important role in the pathological progression of PD. Previous studies have shown that enhancing autophagy can significantly reduce neuroinflammation in PD (18,19). BAG3 is a molecule related to autophagy, and to verify whether it could regulate the level of autophagy in the neuroinflammation of PD, we detected expression of autophagy-related proteins at 3 days after injection of LPS (Figure 5A-5F). LPS increased expression of p62, and the level of p62 in the LPS + BAG3-AAV group was significantly lower than in the LPS + Con-AAV group. LPS inhibited expression of Beclin1, and BAG3 prevented this effect. In addition, there was no significant difference between autophagy related protein (ATG5) and light chain 3 (LC3). Our results show that LPS-induced neuroinflammation leads to autophagy disorder, and BAG3 can reverse this.
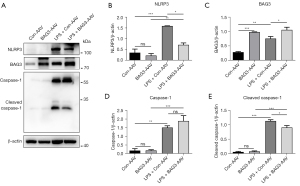
Overexpression of BAG3 reduces LPS-induced pyroptosis
Pyroptosis is a new form of programmed cell death characterized by its dependence on caspase-1, the NLRP3 inflammasome, and the release of a large number of inflammatory cytokines (20). Many studies have reported that pyroptosis plays an important role in PD, and inhibiting the activation of the NLRP3 inflammasomes can protect dopaminergic neurons (21,22). We have verified in vitro that BAG3 is involved in activation of the NLRP3 inflammasomes in BV2 cells induced by LPS, and to verify whether BAG3 plays this role in PD mice, we detected expression of NLRP3 and caspase-1 in the striatum of mice (Figure 6A-6E). Western blotting showed the expression of NLRP3, caspase-1, and cleaved caspase-1 in the LPS + Con-AAV group was significantly higher than in the Con-AAV group, indicating LPS induced pyroptosis in the striatum. The LPS + BAG3-AAV group showed a strong anti-inflammatory effect of BAG3, and compared with the LPS + Con-AAV group, overexpression of BAG3 inhibited activation of NLRP3 and reduced expression of cleaved caspase-1, indicating BAG3 can inhibit pyroptosis caused by LPS in mice.
Discussion
Although the potential mechanism remains unclear, studies have shown that neuroinflammation is an important mechanism of pathological progression in PD (23-25). Microglia play an important role in central nervous system inflammation in PD (26), and there are many activated microglia in the substantia nigra of PD patients (27). Persistent neuroinflammation caused by these activated microglia is a necessary factor in PD, and NLRP3 inflammasomes are intracellular pro-inflammatory pattern recognition receptors that can trigger and spread inflammation (28). These cell complexes are well characterized in the innate immune system, and their activity has been reported in microglia (29). Activation of NLRP3 inflammasomes in microglia often aggravates the loss of dopaminergic neurons (22,30), and they appear necessary for microglia-mediated neuroinflammation and dopaminergic neuronal loss, which has been confirmed in a variety of PD models such as MPTP, LPS, rotenone, and 6-OHDA models (31-34).
BAG3 is a protein composed of 575 amino acids which is highly expressed in the heart (35), and may play an important role in the brain (36). In this study, we demonstrated that overexpression of BAG3 in the striatum with BAG3-AAV can significantly reduce neuroinflammation and dopaminergic neuronal loss induced by LPS injection and can inhibit microglial activation and the inflammatory cytokines TNF-α and IL-1β. Further studies confirmed BAG3 inhibited the activation of NLRP3 inflammasomes in microglia, which slowed down the loss of dopaminergic neurons induced by LPS. In vitro studies also found that knockdown of BAG3 increased the expression of inflammatory cytokines and promoted activation of NLRP3 inflammasomes induced by LPS, which was consistent with our findings in vivo, and indicated BAG3 regulates the activation of NLRP3 inflammasomes to regulate the occurrence of neuroinflammation in PD.
Autophagy is a lysosome-dependent degradation pathway. Dysfunctional autophagy leads to protein accumulation and neuronal loss, and is increasingly considered to play a key role in PD (37). BAG3 plays an important role in autophagy and mitochondrial autophagy, and is seen to deliver ubiquitinated and misfolded proteins by forming complexes with HSP70 and HSPB8, which is a special and selective process (38). In our previous study, we reported that BAG3 promoted autophagy to clear the pathogenic protein α-synuclein in PD, and that it regulated autophagy of PD (10).
The regulatory effect of autophagy on inflammation is a recent discovery. Enhancing autophagy in an inflammatory environment can significantly inhibit inflammation and protect dopaminergic neurons in PD (16,39,40). Based on one of our previous studies and the important role of BAG3 in autophagy (10), we hypothesized that BAG3 regulates PD pathology by regulating autophagy in PD-related inflammation and validates the specific molecular mechanism. We found p62 expression increased and Beclin1 expression decreased in the LPS-induced PD mouse model, which indicates autophagy is inhibited in the LPS inflammatory environment, which is consistent with previous reports. p62 has an important role in autophagy, and its overexpression promotes inflammation (41). We overexpressed BAG3 in the striatum and found it played a role in promoting autophagy by restoring the decreased expression of Beclin1 induced by LPS and inhibiting the increase of p62. Our results show BAG3 regulates autophagy in PD inflammation.
In summary, our study proposed the mechanism of action of BAG3 in PD neuroinflammation and the results show BAG3 can inhibit the occurrence of cell pyroptosis in microglia and restore expression of autophagy suppressed under inflammatory conditions.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81801259), Jiangsu Provincial Key R&D Program (No. BE2018658), Suzhou Technology Development Programme (No. SLJ2021010), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5159/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5159/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5159/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 202206A0353) granted by the Ethics Committee of Soochow University, in compliance with the institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blesa J, Foffani G, Dehay B, et al. Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci 2022;23:115-28. [Crossref] [PubMed]
- Tan EK, Chao YX, West A, et al. Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat Rev Neurol 2020;16:303-18. [Crossref] [PubMed]
- Zhou Q, Zhang Y, Lu L, et al. Copper induces microglia-mediated neuroinflammation through ROS/NF-κB pathway and mitophagy disorder. Food Chem Toxicol 2022;168:113369. [Crossref] [PubMed]
- Hirsch EC, Standaert DG. Ten Unsolved Questions About Neuroinflammation in Parkinson's Disease. Mov Disord 2021;36:16-24. [Crossref] [PubMed]
- Hou X, Watzlawik JO, Fiesel FC, et al. Autophagy in Parkinson's Disease. J Mol Biol 2020;432:2651-72. [Crossref] [PubMed]
- Liang Z, Zhang S, Zou Z, et al. Functional characterization of BAG3 in orange-spotted grouper (Epinephelus coioides) during viral infection. Fish Shellfish Immunol 2022;122:465-75. [Crossref] [PubMed]
- Chillappagari S, Schwarz J, Kesireddy V, et al. Therapeutic induction of Bcl2-associated athanogene 3-mediated autophagy in idiopathic pulmonary fibrosis. Clin Transl Med 2022;12:e935. [Crossref] [PubMed]
- Zhao S, Wang JM, Yan J, et al. BAG3 promotes autophagy and glutaminolysis via stabilizing glutaminase. Cell Death Dis 2019;10:284. [Crossref] [PubMed]
- De Marco M, Basile A, Iorio V, et al. Role of BAG3 in cancer progression: A therapeutic opportunity. Semin Cell Dev Biol 2018;78:85-92. [Crossref] [PubMed]
- Cao YL, Yang YP, Mao CJ, et al. A role of BAG3 in regulating SNCA/α-synuclein clearance via selective macroautophagy. Neurobiol Aging 2017;60:104-15. [Crossref] [PubMed]
- Colvin TA, Gabai VL, Gong J, et al. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer Res 2014;74:4731-40. [Crossref] [PubMed]
- Nivon M, Abou-Samra M, Richet E, et al. NF-κB regulates protein quality control after heat stress through modulation of the BAG3-HspB8 complex. J Cell Sci 2012;125:1141-51. [Crossref] [PubMed]
- Badanjak K, Fixemer S, Smajić S, et al. The Contribution of Microglia to Neuroinflammation in Parkinson's Disease. Int J Mol Sci 2021;22:4676. [Crossref] [PubMed]
- Nguyen LTN, Nguyen HD, Kim YJ, et al. Role of NLRP3 Inflammasome in Parkinson's Disease and Therapeutic Considerations. J Parkinsons Dis 2022;12:2117-33. [Crossref] [PubMed]
- Zheng R, Ruan Y, Yan Y, et al. Melatonin Attenuates Neuroinflammation by Down-Regulating NLRP3 Inflammasome via a SIRT1-Dependent Pathway in MPTP-Induced Models of Parkinson's Disease. J Inflamm Res 2021;14:3063-75. [Crossref] [PubMed]
- Dong AQ, Yang YP, Jiang SM, et al. Pramipexole inhibits astrocytic NLRP3 inflammasome activation via Drd3-dependent autophagy in a mouse model of Parkinson's disease. Acta Pharmacol Sin 2022; Epub ahead of print. [Crossref] [PubMed]
- Yao L, Ye Y, Mao H, et al. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson's disease. J Neuroinflammation 2018;15:13. [Crossref] [PubMed]
- Berglund R, Guerreiro-Cacais AO, Adzemovic MZ, et al. Microglial autophagy-associated phagocytosis is essential for recovery from neuroinflammation. Sci Immunol 2020;5:eabb5077. [Crossref] [PubMed]
- Tu HY, Yuan BS, Hou XO, et al. α-synuclein suppresses microglial autophagy and promotes neurodegeneration in a mouse model of Parkinson's disease. Aging Cell 2021;20:e13522. [Crossref] [PubMed]
- Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 2017;42:245-54. [Crossref] [PubMed]
- Panicker N, Kam TI, Wang H, et al. Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson's disease. Neuron 2022;110:2422-37.e9. [Crossref] [PubMed]
- Qin Y, Qiu J, Wang P, et al. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson's disease. Brain Behav Immun 2021;91:324-38. [Crossref] [PubMed]
- Li Y, Xia Y, Yin S, et al. Targeting Microglial α-Synuclein/TLRs/NF-kappaB/NLRP3 Inflammasome Axis in Parkinson's Disease. Front Immunol 2021;12:719807. [Crossref] [PubMed]
- La Vitola P, Balducci C, Baroni M, et al. Peripheral inflammation exacerbates α-synuclein toxicity and neuropathology in Parkinson's models. Neuropathol Appl Neurobiol 2021;47:43-60. [Crossref] [PubMed]
- Kwon OC, Song JJ, Yang Y, et al. SGK1 inhibition in glia ameliorates pathologies and symptoms in Parkinson disease animal models. EMBO Mol Med 2021;13:e13076. [Crossref] [PubMed]
- Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov Disord 2011;26:1049-55. [Crossref] [PubMed]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol 2009;8:382-97. [Crossref] [PubMed]
- Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol 2021;18:2114-27. [Crossref] [PubMed]
- Wu AG, Zhou XG, Qiao G, et al. Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res Rev 2021;65:101202. [Crossref] [PubMed]
- Haque ME, Akther M, Jakaria M, et al. Targeting the microglial NLRP3 inflammasome and its role in Parkinson's disease. Mov Disord 2020;35:20-33. [Crossref] [PubMed]
- Martinez EM, Young AL, Patankar YR, et al. Editor's Highlight: Nlrp3 Is Required for Inflammatory Changes and Nigral Cell Loss Resulting From Chronic Intragastric Rotenone Exposure in Mice. Toxicol Sci 2017;159:64-75. [Crossref] [PubMed]
- Yan Y, Jiang W, Liu L, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015;160:62-73. [Crossref] [PubMed]
- Qiao C, Zhang LX, Sun XY, et al. Caspase-1 Deficiency Alleviates Dopaminergic Neuronal Death via Inhibiting Caspase-7/AIF Pathway in MPTP/p Mouse Model of Parkinson's Disease. Mol Neurobiol 2017;54:4292-302. [Crossref] [PubMed]
- Mao Z, Liu C, Ji S, et al. The NLRP3 Inflammasome is Involved in the Pathogenesis of Parkinson's Disease in Rats. Neurochem Res 2017;42:1104-15. [Crossref] [PubMed]
- Feldman AM, Gordon J, Wang J, et al. BAG3 regulates contractility and Ca(2+) homeostasis in adult mouse ventricular myocytes. J Mol Cell Cardiol 2016;92:10-20. [Crossref] [PubMed]
- Li X, Lin G, Liu T, et al. Postnatal development of BAG3 expression in mouse cerebral cortex and hippocampus. Brain Struct Funct 2021;226:2629-50. [Crossref] [PubMed]
- Ghavami S, Shojaei S, Yeganeh B, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 2014;112:24-49. [Crossref] [PubMed]
- Kirk JA, Cheung JY, Feldman AM. Therapeutic targeting of BAG3: considering its complexity in cancer and heart disease. J Clin Invest 2021;131:149415. [Crossref] [PubMed]
- Pupyshev AB, Tenditnik MV, Ovsyukova MV, et al. Restoration of Parkinson's Disease-Like Deficits by Activating Autophagy through mTOR-Dependent and mTOR-Independent Mechanisms in Pharmacological and Transgenic Models of Parkinson's Disease in Mice. Bull Exp Biol Med 2021;171:425-30. [Crossref] [PubMed]
- Zhang Q, Zhou J, Shen M, et al. Pyrroloquinoline Quinone Inhibits Rotenone-Induced Microglia Inflammation by Enhancing Autophagy. Molecules 2020;25:4359. [Crossref] [PubMed]
- Xin X, Wang C, Lin Z, et al. Inflammatory-Related P62 Triggers Malignant Transformation of Mesenchymal Stem Cells through the Cascade of CUDR-CTCF-IGFII-RAS Signaling. Mol Ther Nucleic Acids 2018;11:367-81. [Crossref] [PubMed]
(English Language Editor: B. Draper)


