
Astragaloside IV ameliorates spinal cord injury through controlling ferroptosis in H2O2-damaged PC12 cells in vitro
Introduction
Spinal cord injury (SCI) is a temporary or permanent damage to the spinal cord structure and function (1). SCI often causes a number of neurological disorders, including limb pain, numbness, weakness, paralysis, and urinary and fecal disorders, all of which may be life-threatening (2). The etiology of SCI can be classified into traumatic and non-traumatic factors, with the former being the main cause of SCI (3,4). Traumatic SCI refers to acute injury of the spinal cord caused by external physical impacts, such as sports-related injuries, motor vehicle injuries, violence, or falls (5). Non-traumatic SCI refers to injury to the spinal cord in the course of an acute or chronic disease, including infections, degenerative diseases, or tumors (6). Recent epidemiological data revealed that there are about 23 new SCI cases per million people worldwide annually (7). SCI has a high incidence and can cause severe disability, especially in young adults (8). Currently, the treatment modalities of SCI include surgery and nonsurgical therapies, the latter of which mainly includes drug therapy, vascular booster therapy, stem cell therapy, hypothermia therapy, growth factor therapy, and electrical stimulation of the spinal cord (9,10). However, management of SCI patients is associated with high costs and poor curative rates. Therefore, the prevention and treatment of SCI is a challenging global medical and social problem.
Astragalus membranaceus (Ast) is a Traditional Chinese medicine that is used for tonifying middle-Jiao and Qi, inducing diuresis to alleviate edema, promoting the elimination of toxins, and the production of new tissues (11). Astragaloside IV (AS-IV), the main active ingredients of Ast, has a powerful antioxidant effect by removing free radicals and reducing lipid peroxidation (12). To date, AS-IV has been demonstrated to have multiple pharmacological effects, including anti-diabetes, anti-hypertension, myocardial protection, anti-heart failure, anti-inflammation, and anti-infarction (12,13). Furthermore, both in vitro and in vivo studies have shown that AS-IV can distinctly reduce the levels of reactive oxygen species (ROS) to alleviate oxidative damage (14,15). There is an overproduction of ROS after SCI and this facilitates the cascade of secondary injuries (16). ROS clearance can prevent or relieve the secondary injury after SCI (17). Therefore, we hypothesized that AS-IV may have certain protective effects against SCI. However, the specific functions and mechanisms of AS-IV in SCI remain to be fully elucidated.
In the past decade, study has shown that apoptosis, autophagy, necrosis and pyroptosis can regulate the occurrence and development of SCI (18). Lately, as a new term, ferroptosis has been paid more and more attention in SCI (19). Ferroptosis is an iron-dependent form of non-apoptotic cell death driven by excessive iron accumulation and lipid peroxidation (20). Ferroptotic cells are characterized by mitochondrial abnormalities such as swelling, increased membrane density, crest loss, and rupture of the outer membrane (21). Recently, some studies have shown that ferrostatin-1, an inhibitor of ferroptosis, improved recovery of spinal cord function after injury (22,23). Therefore, ferroptosis may be a potential therapeutic target for SCI. In fact, report suggests that inhibiting iron death is helpful to alleviate spinal cord injury (24). For example, Ge et al. find that Zinc inhibits ferroptosis and alleviates spinal cord injury through Nrf2/GPX4 pathway (25). In addition, AS-IV has been shown to reduce ROS production and improve oxidative stress (26). However, whether AS-IV can alleviate SCI through ferroptosis and the regulatory mechanisms involved remains to be elucidated. Therefore, this current study established a SCI model in vitro by H2O2 pretreatment of PC12 pheochromocytoma cells, to observe the function of AS-IV in SCI. The underlying mechanisms of AS-IV in H2O2-damaged PC12 cells were analyzed. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5196/rc).
Methods
Cell culture
The PC12 pheochromocytoma cell line was obtained from the National Infrastructure of Cell Line Resource (Beijing, China). Cells were grown in RPMI1640 medium (Gibco) supplemented with 5% fetal bovine serum (FBS, Gibco, Cat. No. 10270) at 37 ℃ in a 5% CO2 incubator. To induce a nerve cell phenotype, cells were treated with 0.5 µg/mL nerve growth factor (NGF) for 5 days.
Cell treatment
PC12 cells were incubated with 0, 0.01, 0.1, 1, 10, or 100 µM AS-IV (HPLC ≥98.0%, Cat No. JZ16042403) for 0, 24, 48, 72, and 96 h. Negative control (NC) small interfering (si)RNA and si-transcription factor EB (TFEB) were obtained from GenePharma (Shanghai, China). The H2O2-treated PC12 cell model was established using 300 µmol/L H2O2. The H2O2-damaged PC12 cells were treated with 0.1 µM AS-IV and 5 µM FIN56 (AbMole, USA), which is a specific inducer of ferroptosis. Cells were transfected with NC siRNA and si-TFEB using Lipofectamine 3000 (Life Technologies).
Cell counting kit 8 (CCK-8) assay
After specific treatment, PC12 cells were harvested and seeded into 96-well plates at 5,000 cells/well and maintained at 37 ℃ for 24 h. Cells were then incubated with 10 µL CCK-8 for 1 h at 37 ℃. The optical density (OD) value at 450 nm was determined using a microplate reader.
Caspase-3 activity assay
Caspase-3 activity was determined using the Caspase-3 Assay Kit (ab39401, Abcam). Briefly, proteins were harvested from PC12 cells and the concentration was adjusted. Caspase-3 activity was then determined in accordance with the manufacturer’s instructions.
Measurement of intracellular reactive oxygen species
To determine the levels of ROS, PC12 cells were harvested and exposed to 10 µM DCFDA (abcam, USA) in serum-free medium at 37 ℃ for 45 min in the dark. Cells were then washed with phosphate buffered saline (PBS) and the intensity of fluorescent DCF at 485/535 nm (ex/em) was measured using a fluorescence microscope. Additionally, flow cytometry (FACScan, USA) was performed to determine the levels of ROS in PC12 cells. The data was analyzed using CELL Quest 3.0 software.
Enzyme-linked immunosorbent assay (ELISA)
PC12 cells were harvested and seed into 6-well plates at 1×104 cells/well. After treatment with H2O2, AS-IV, FIN56, or si-TFEB, the cell supernatant was harvested and the lactate dehydrogenase (LDH) concentrations were determined using the LDH ELISA Kit (Roche, Germany) as per the manufacturer’s protocols.
Transmission electron microscopy (TEM)
Treated PC12 cells were harvested and incubated with 2% glutaraldehyde (Cat. no. 354400) overnight at 4 ℃. The cells were then fixed with 1% osmic acid at 4 ℃ for 2 h, followed by washing and staining with uranyl acetate (Electron Microscopy Sciences, Cat. No. 22,400). Cells were then dehydrated with 70%, 80%, 90%, and 100% acetone, sequentially. After pre-embedding, the cells were embedded with Epon812 epoxy resin (Hede Biotechnology Co., Ltd., Beijing, China) and stained with toluidine blue (Solarbio). Following sectioning, the results were observed under a TEM (JEOL, Tokyo, JEM-1011).
Western blot
To extract the total protein, treated PC12 cells were lysed using RIPA buffer (Cell Signaling Technology) supplemented with protease inhibitor (Sigma-Aldrich, Cat. no. P8340). The nuclear and cytoplasmic proteins were acquired by applying Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific). After protein quantification, the samples were separated using sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred onto polyvinylidene fluoride (PVDF) membranes. After blocking by skin milk, the proteins were immersed in an appropriate dilution of the primary antibodies, and the corresponding secondary antibody. Individual protein bands were visualized using the electrochemiluminescence (ECL) kit (Amersham Biosciences). The primary antibodies used were SLC7A11 (Abcam, ab175186), GPX4 (Abcam, ab125066), TFEB (Abcam, ab270614), GAPDH (Cell Signaling Technology), and horse radish peroxidase (HRP)-labeled secondary antibody (Cell Signaling Technology, Bioke).
Immunofluorescence assay
The treated PC12 cells were seeded in a 6-well plate with coverslips and incubated at 37 ℃ for 6 h. After washing with pre-cooled PBS, PC12 cells were fixed with 40 g/L paraformaldehyde for 20 min, and treated with Triton X-100 (10 mL/L) for 15 min. After blocking with 100 mL/L goat serum for 30 min, the cells were incubated with anti-TFEB (1:200, Abcam, ab270614) at 4 ℃ overnight, followed by the CY5-labeled secondary antibody (1:200, Abcam) at 37 ℃ for 1 h. Cells were then stained with 100 µL DAPI (100 g/L) for 5 min in the dark. TFEB expression was visualized and photographed under a fluorescence microscope.
Statistical analysis
The data herein are presented as mean ± standard deviation (SD) based on 3 replicates, and analyzed using one-way analysis of variance (ANOVA). All statistical analyses were performed using the SPSS20.0 software. A P value <0.05 was considered statistically significant.
Results
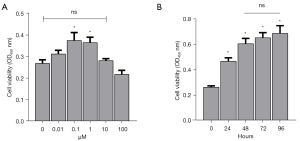
The impacts of AS-IV on the viability of the cultured PC12 cells
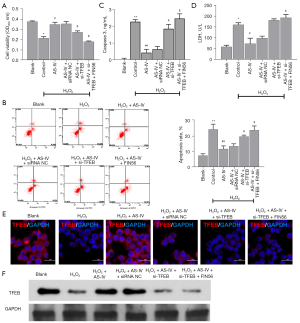
To investigate the influence of AS-IV on PC12 cells, cell viability and proliferation were examined using the CCK-8 assay after incubation with 0, 0.01, 0.1, 1.0, 10, and 100 µM AS-IV for 24–96 h. As displayed in Figure 1A, 0.1 µM and 1 µM AS-IV significantly increased the viability of PC12 cells compared to untreated cells. Indeed, the enhanced cell viability was more pronounced with 0.1 µM AS-IV than with 1.0 µM AS-IV. AS-IV (0.1 µM) also significantly enhanced the proliferative activity of PC12 cells after treatment for 24–96 h, especially after 48, 72, and 96 h (Figure 1B). After a comprehensive analysis, a concentration of 0.1 µM AS-IV was adopted for the all subsequent experiments with an incubation time of 48 h.
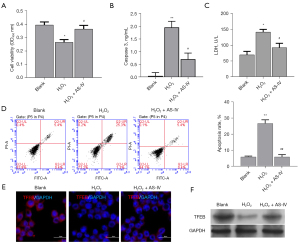
AS-IV protects PC12 cells against injury induced by H2O2 and enhances TFEB expression
Accumulating evidence suggests that an excess of ROS plays a crucial role in secondary injury cascades of SCI (27). To further investigate the protective role of AS-IV on SCI, 300 µmol/L H2O2 was used to induce the OS (oxidative stress) of PC12 cells, and the H2O2-treated PC12 cells were then incubated with 0.1 µM AS-IV. Cell viability was markedly reduced in the H2O2-treated cells compared to untreated control cells. However, incubation with AS-IV significantly increased the viability of H2O2-treated PC12 cells (Figure 2A). In addition, H2O2 markedly induced the apoptosis of PC12 cells, and this could be reversed by treatment with AS-IV (Figure 2B). Furthermore, H2O2 significantly elevated the levels of caspase 3 and LDH in PC12 cells, and this was ameliorated by treatment with AS-IV (Figure 2C,2D). H2O2-treated cells showed suppressed expression of TFEB compared to control cells, and its expression was reversed after AS-IV administration (Figure 2E,2F). These results demonstrated that AS-IV effectively alleviated the H2O2-induced effects on PC12 cells.
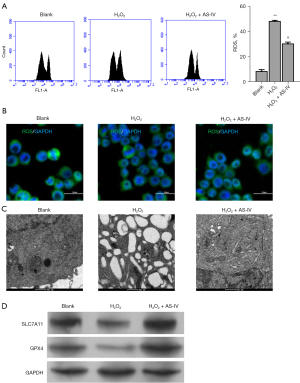
AS-IV suppressed ferroptosis in H2O2-treated PC12 cells
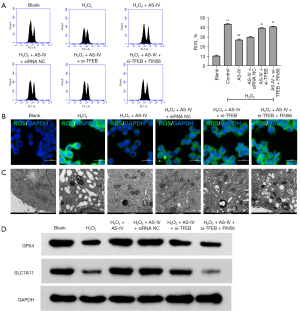
The role of ferroptosis in the protective effects of AS-IV on H2O2-treated PC12 cells was examined. Flow cytometry showed that AS-IV reduced the ROS production induced in H2O2-treated PC12 cells (Figure 3A,3B). TEM demonstrated that the mitochondria in the H2O2 group were small and atrophied, and the mitochondrial ridges were decreased or even absent. Interestingly, AS-IV reversed the mitochondrial morphology observed in H2O2-treated cells (Figure 3C). To examine the role of ferroptosis in the protective effects of AS-IV in H2O2-treated PC12 cells, the expression of SLC7A11 and GPX4 was measured by Western blot analysis. As shown in Figure 3D, the expression of SLC7A11 and GPX4 was suppressed by H2O2 treatment, and recovered by AS-IV (Figure 3D). These results suggested that AS-IV exerted a protective action by inhibiting ferroptosis in H2O2-treated PC12 cells.
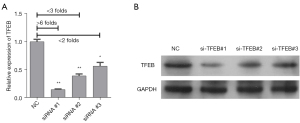
Verification of TFEB knockdown in PC12 cells
To further determine whether the protective action of AS-IV on SCI is associated with TFEB, PC12 cells were transfected with NC, si-TFEB#1, si-TFEB#2, or si-TFEB#3. The results of quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot verified that TFEB was dramatically downregulated in all cells transfected with the si-TFEBs compared to the NC group, in particular, si-TFEB#1 (Figure 4A,4B). Therefore, the si-TFEB#1 was used to transfect PC12 cells for all subsequent experiments.
TFEB-mediated ferroptosis is involved in H2O2-induced cell injury
We further explored whether the remission of H2O2-induced SCI by AS-IV is mediated through regulation of TFEB and ferroptosis. H2O2-treated PC12 cells were incubated with 0.1 µM AS-IV, si-TFEBs, and/or 5 µM FIN56. Knockdown of TFEB reversed the protective effect of AS-IV on cell viability in H2O2-treated PC12 cells, and FIN56 effectively enhanced the effect of si-TFEB (Figure 5A). Similarly, knockdown of TFEB reversed the protective effect of AS-IV on the rate of cell apoptosis in H2O2-treated PC12 cells. The combination of si-TFEB and FIN56 resulted in a further increase in the cell apoptosis rate compared with si-TFEB alone (Figure 5B). Furthermore, knockdown of TFEB was effective in ameliorating the decreased expression of caspase 3 and LDH caused by AS-IV in H2O2-treated PC12 cells. FIN56 further enhanced the effect of si-TFEB on caspase 3 expression in H2O2-treated PC12 cells treated with AS-IV (Figure 5C,5D). Immunofluorescence assays showed that TFEB expression was downregulated by TFEB siRNA, and its expression was further suppressed by the ferroptosis inducer, FIN56 (Figure 5E,5F). These results suggested that TFEB is required for the regulatory effects of AS-IV on proliferation and apoptosis of H2O2-damaged PC12 cells.
TFEB knockdown reversed the protective effect of AS-IV by mediating ferroptosis in H2O2-treated PC12 cells
The flow cytometry and DCFH-DA data demonstrated that AS-IV remarkably downregulated the levels of ROS, while TFEB silencing had the opposite effect by further increasing ROS accumulation. FIN56 enhanced the effects of si-TFEB (Figure 6A,6B). TEM observations revealed that si-TFEB reversed the protective effect of AS-IV on the mitochondria, and the addition of FIN56 further aggravated the mitochondrial abnormalities (Figure 6C). Transfection of si-TFEBs impaired the expression of SLC7A11 and GPX4, and this was further reduced by FIN56 (Figure 6D). Taken together, these results suggested that AS-IV alleviated H2O2-induced damage to PC12 cells through promoting TFEB expression and the subsequent suppression of ferroptosis.
Discussion
SCI is a common presentation for spinal surgery, and its pathological process includes primary and secondary injuries (28). Secondary injury is a series of physiological and biochemical reactions such as ischemia, edema, and electrolyte disturbance on the basis of the primary injury (29,30). Ischemia, hypoxia, and inflammation can cause the production and release of ROS (31-33), which can lead to oxidative damage of local tissues, as well as apoptosis and necrosis of nerve cells (34). Therefore, the development of effective drugs to prevent the secondary death of spinal cord neurons is crucial.
Chinese herb derived active components, such as triptolide and salvianic acid B, have been proven to alleviate spinal cord injury through multiple pathways (35,36). AS-IV, as one of the main active components of Ast, has a wide range of pharmacological activities on the central nervous system (CNS) (12). Studies show that AS-IV has no obvious hepatotoxicity and nephrotoxicity (37,38). A large number of studies have shown that AS-IV can effectively delay the aging of body cells, promote proliferation, resist inflammation and apoptosis, and protect against oxidative stress of neuronal cells (15,39). In this current study, an oxidative stress injury model was established by pretreatment of PC12 cells with H2O2, and the effects of AS-IV were examined. The optimal concentration and exposure time of AS-IV in PC12 cells was determined to be 1.0 M and 48 h, respectively. AS-IV significantly enhanced proliferation, and inhibited apoptosis and ferroptosis in H2O2-damaged PC12 cells, and this effect was reversed by FIN56. This suggested that the ferroptosis pathway is involved in the protective effect of AS-IV on SCI.
Studies have demonstrated that ferroptosis signaling seriously affects SCI. After SCI, massive hemorrhage, cell rupture, hemolysis, erythrocyte aggregation, and iron overload occur in the spinal cord, and stress increases the production of ROS and the excitotoxicity of glutamate (40,41). All these factors can stimulate ferroptosis (42). Some ferroptosis inhibitors, such as SRS 16-86 (43), lipro-1 (44), and deferoxamine (40) may be potential drugs for the treatment of SCI. FIN56 is a ferroptosis inducer and triggers ferroptosis via inducing the degradation of GPX4 (45). Some reports have shown that ferroptosis is closely related to lysosomal membrane permeabilization (46). The microphthalmia-associated transcription factor (MITF) family include MITF, TFEB, TFE3 and transcription factor EC (TFCE) (47). Among them, TFEB can be expressed in a variety of tissues (48). Since TFEB is reported to be essential in lysosomal biogenesis (49) and clearance of oxidative stress (50), we hypothesized that TFEB might participate in the ferroptosis process of SCI.
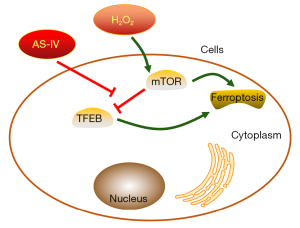
The current investigation demonstrated that AS-IV notably promoted the expression of TFEB in H2O2-damaged PC12 cells. In addition, TFEB was involved in the regulatory effects of AS-IV on the proliferation and apoptosis of H2O2-treated PC12 cells. Moreover, AS-IV-mediated expression of TFEB was remarkably attenuated by TFEB knockdown, and the combination of FIN56 and si-TFEB further attenuated the effects of AS-IV on H2O2-damaged PC12 cells (Figure 7).
Conclusions
AS-IV can protect PC12 cells against oxidative injury mediated by H2O2 via expression of TFEB and the subsequent suppression of ferroptosis. AS-IV is expected to inhibit the apoptosis of nerve cells caused by spinal cord injury and induce the generation of spinal cord scar. At present, the clinical development and promotion of AS-IV has been stagnant due to the low output of astragalus and the difficulty in chemical synthesis. Therefore, there is still a lack of corresponding clinical data support. The researchers believe that AS-IV derivatives with high water solubility and bioavailability should be concerned. While, this requires more in-depth research, development and utilization.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5196/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-5196/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-5196/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Eckert MJ, Martin MJ. Trauma: Spinal Cord Injury. Surg Clin North Am 2017;97:1031-45. [Crossref] [PubMed]
- Hamid R, Averbeck MA, Chiang H, et al. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J Urol 2018;36:1517-27. [Crossref] [PubMed]
- Rouanet C, Reges D, Rocha E, et al. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuropsiquiatr 2017;75:387-93. [Crossref] [PubMed]
- Seif M, David G, Huber E, et al. Cervical Cord Neurodegeneration in Traumatic and Non-Traumatic Spinal Cord Injury. J Neurotrauma 2020;37:860-7. [Crossref] [PubMed]
- Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers 2017;3:17018. [Crossref] [PubMed]
- Clark JM, Marshall R. Nature of the Non-traumatic Spinal Cord Injury Literature: A Systematic Review. Top Spinal Cord Inj Rehabil 2017;23:353-67. [Crossref] [PubMed]
- Chuang CH, Chen CH, Bai CH, et al. Risk factors associated with newly psychiatric disorder in spinal cord injury: A retrospective cohort study. J Clin Nurs 2018;27:e1038-e47. [Crossref] [PubMed]
- Ge L, Arul K, Mesfin A. Spinal Cord Injury From Spinal Tumors: Prevalence, Management, and Outcomes. World Neurosurg 2019;122:e1551-e6. [Crossref] [PubMed]
- Karsy M, Hawryluk G. Modern Medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep 2019;19:65. [Crossref] [PubMed]
- Jendelova P. Therapeutic Strategies for Spinal Cord Injury. Int J Mol Sci 2018;19:3200. [Crossref] [PubMed]
- Auyeung KK, Han QB, Ko JK. Astragalus membranaceus: A Review of its Protection Against Inflammation and Gastrointestinal Cancers. Am J Chin Med 2016;44:1-22. [Crossref] [PubMed]
- You LZ, Lin YX, Fang ZH, et al. Research advances on astragaloside-IV in treatment of diabetes mellitus and its complications pharmacological effects. Zhongguo Zhong Yao Za Zhi 2017;42:4700-6. [PubMed]
- Zhang J, Wu C, Gao L, et al. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol 2020;87:89-112. [Crossref] [PubMed]
- Jiang M, Ni J, Cao Y, et al. Astragaloside IV Attenuates Myocardial Ischemia-Reperfusion Injury from Oxidative Stress by Regulating Succinate, Lysophospholipid Metabolism, and ROS Scavenging System. Oxid Med Cell Longev 2019;2019:9137654. [Crossref] [PubMed]
- Yang C, Mo Y, Xu E, et al. Astragaloside IV ameliorates motor deficits and dopaminergic neuron degeneration via inhibiting neuroinflammation and oxidative stress in a Parkinson's disease mouse model. Int Immunopharmacol 2019;75:105651. [Crossref] [PubMed]
- Chen X, Cui J, Zhai X, et al. Inhalation of Hydrogen of Different Concentrations Ameliorates Spinal Cord Injury in Mice by Protecting Spinal Cord Neurons from Apoptosis, Oxidative Injury and Mitochondrial Structure Damages. Cell Physiol Biochem 2018;47:176-90. [Crossref] [PubMed]
- Jia Z, Zhu H, Li J, et al. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012;50:264-74. [Crossref] [PubMed]
- Zhu N, Ruan J, Yang X, et al. Triptolide improves spinal cord injury by promoting autophagy and inhibiting apoptosis. Cell Biol Int 2020;44:785-94. [Crossref] [PubMed]
- Rong Y, Fan J, Ji C, et al. USP11 regulates autophagy-dependent ferroptosis after spinal cord ischemia-reperfusion injury by deubiquitinating Beclin 1. Cell Death Differ 2022;29:1164-75. [Crossref] [PubMed]
- Tang D, Chen X, Kang R, et al. Ferroptosis: molecular mechanisms and health implications. Cell Res 2021;31:107-25. [Crossref] [PubMed]
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060-72. [Crossref] [PubMed]
- Ge H, Xue X, Xian J, et al. Ferrostatin-1 Alleviates White Matter Injury Via Decreasing Ferroptosis Following Spinal Cord Injury. Mol Neurobiol 2022;59:161-76. [Crossref] [PubMed]
- Wei N, Lu T, Yang L, et al. Lipoxin A4 protects primary spinal cord neurons from Erastin-induced ferroptosis by activating the Akt/Nrf2/HO-1 signaling pathway. FEBS Open Bio 2021;11:2118-26. [Crossref] [PubMed]
- Chen Y, Liu S, Li J, et al. The Latest View on the Mechanism of Ferroptosis and Its Research Progress in Spinal Cord Injury. Oxid Med Cell Longev 2020;2020:6375938. [Crossref] [PubMed]
- Ge MH, Tian H, Mao L, et al. Zinc attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury by activating Nrf2/GPX4 defense pathway. CNS Neurosci Ther 2021;27:1023-40. [Crossref] [PubMed]
- Zhang Y, Mao XD, Cao AL, et al. Astragaloside IV prevents endothelial dysfunction by improving oxidative stress in streptozotocin-induced diabetic mouse aortas. Exp Ther Med 2021;22:1197. [Crossref] [PubMed]
- Sullivan PG, Krishnamurthy S, Patel SP, et al. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma 2007;24:991-9. [Crossref] [PubMed]
- Sweis R, Biller J. Systemic Complications of Spinal Cord Injury. Curr Neurol Neurosci Rep 2017;17:8. [Crossref] [PubMed]
- Vaidyanathan S, Mansour P, Soni BM, et al. Should spinal cord clinicians be proactive in preventing spinal cord injuries and decreasing secondary complications caused by spinal cord injury? Spinal Cord 2003;41:475-7. [Crossref] [PubMed]
- Schwartzbauer G, Stein D. Critical Care of Traumatic Cervical Spinal Cord Injuries: Preventing Secondary Injury. Semin Neurol 2016;36:577-85. [Crossref] [PubMed]
- Qiu Z, He Y, Ming H, et al. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J Diabetes Res 2019;2019:8151836. [Crossref] [PubMed]
- Liu Z, Qu M, Yu L, et al. Artesunate Inhibits Renal Ischemia-Reperfusion-Mediated Remote Lung Inflammation Through Attenuating ROS-Induced Activation of NLRP3 Inflammasome. Inflammation 2018;41:1546-56. [Crossref] [PubMed]
- Markowska A, Huczyński A, Kojs Z, et al. Hypoxia and its importance in the course of gynaecological cancers. European Journal of Gynaecological Oncology 2019;40:711-3.
- Li J, Wang Q, Cai H, et al. FGF1 improves functional recovery through inducing PRDX1 to regulate autophagy and anti-ROS after spinal cord injury. J Cell Mol Med 2018;22:2727-38. [Crossref] [PubMed]
- Fu J, Fan HB, Guo Z, et al. Salvianolic acid B attenuates spinal cord ischemia-reperfusion-induced neuronal injury and oxidative stress by activating the extracellular signal-regulated kinase pathway in rats. J Surg Res 2014;188:222-30. [Crossref] [PubMed]
- Huang Y, Zhu N, Chen T, et al. Triptolide Suppressed the Microglia Activation to Improve Spinal Cord Injury Through miR-96/IKKβ/NF-κB Pathway. Spine (Phila Pa 1976) 2019;44:E707-14. [Crossref] [PubMed]
- Yu SY, Ouyang HT, Yang JY, et al. Subchronic toxicity studies of Radix Astragali extract in rats and dogs. J Ethnopharmacol 2007;110:352-5. [Crossref] [PubMed]
- Gui D, Guo Y, Wang F, et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One 2012;7:e39824. [Crossref] [PubMed]
- Yu W, Lv Z, Zhang L, et al. Astragaloside IV reduces the hypoxia-induced injury in PC-12 cells by inhibiting expression of miR-124. Biomed Pharmacother 2018;106:419-25. [Crossref] [PubMed]
- Derry PJ, Vo ATT, Gnanansekaran A, et al. The Chemical Basis of Intracerebral Hemorrhage and Cell Toxicity With Contributions From Eryptosis and Ferroptosis. Front Cell Neurosci 2020;14:603043. [Crossref] [PubMed]
- Kang JH, Kim MH, Lee HJ, et al. Peroxiredoxin 4 attenuates glutamate-induced neuronal cell death through inhibition of endoplasmic reticulum stress. Free Radic Res 2020;54:207-20. [Crossref] [PubMed]
- Hu X, Xu Y, Xu H, et al. Progress in Understanding Ferroptosis and Its Targeting for Therapeutic Benefits in Traumatic Brain and Spinal Cord Injuries. Front Cell Dev Biol 2021;9:705786. [Crossref] [PubMed]
- Zhang Y, Sun C, Zhao C, et al. Ferroptosis inhibitor SRS 16-86 attenuates ferroptosis and promotes functional recovery in contusion spinal cord injury. Brain Res 2019;1706:48-57. [Crossref] [PubMed]
- Feng Y, Madungwe NB, Imam Aliagan AD, et al. Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem Biophys Res Commun 2019;520:606-11. [Crossref] [PubMed]
- Shimada K, Skouta R, Kaplan A, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 2016;12:497-503. [Crossref] [PubMed]
- Gao H, Bai Y, Jia Y, et al. Ferroptosis is a lysosomal cell death process. Biochem Biophys Res Commun 2018;503:1550-6. [Crossref] [PubMed]
- Kawakami A, Fisher DE. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab Invest 2017;97:649-56. [Crossref] [PubMed]
- Fan Y, Lu H, Liang W, et al. Endothelial TFEB (Transcription Factor EB) Positively Regulates Postischemic Angiogenesis. Circ Res 2018;122:945-57. [Crossref] [PubMed]
- Settembre C, Di Malta C, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science 2011;332:1429-33. [Crossref] [PubMed]
- Tsunemi T, Ashe TD, Morrison BE, et al. PGC-1α rescues Huntington's disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med 2012;4:142ra97. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


