
Use of dexmedetomidine to alleviate intestinal ischemia-reperfusion injury via intestinal microbiota modulation in mice
Introduction
Intestinal ischemia-reperfusion (I/R) injury is a potentially serious process that generally occurs in conditions of intestinal obstruction, abdominal vascular surgery, trauma, or septic shock (1-3). It has been suggested that mucosal barrier dysfunction plays a key role in intestinal I/R injury, allowing bacteria and endotoxins to translocate from the gastrointestinal lumen to distal organs (4,5). This may lead to systemic inflammatory response syndrome, sepsis, and/or multiple organ failure with high mortality and morbidity (6).
The human gastrointestinal tract acts as a reservoir of about 100 trillion microbes, contributing to the maintenance of the mucosal barrier and innate immunity (7). These intestinal microbiotas are recognized as an important health regulator by affecting the host’s metabolism, immune system development, and endocrine function (8-10). Several studies have shown that intestinal I/R injury is a complex pathological condition involving intestinal microbiota alterations. Wang et al. (11) demonstrated that intestinal I/R could induce intestinal microbiota abnormalities and that Firmicutes could quickly respond to it. Conversely, Yoshiya et al. (12) reported that the depletion of commensal bacteria could attenuate intestinal damage. Additionally, germ-free mice were found to show less organ damage following an I/R intestinal injury (13).
During the reperfusion period followed by ischemia, the restoration of blood flow exacerbates injury by inducing inflammatory mediators. As a highly selective α2 adrenergic receptor agonist, dexmedetomidine (DEX) is commonly used as a sedative-hypnotic and analgesic agent that can exert an anti-inflammatory effect (14,15). Our previous study (16) demonstrated DEX’s efficacy in attenuating intestinal I/R injury by inhibiting inflammation, and Shen et al. (17) reported its role in preventing intestinal I/R by directly suppressing the immune response. In addition, several studies have shown the protective effect of DEX on remote organ injuries following intestinal I/R injury (18,19). To date, however, there have been no studies on the alterations in intestinal microbiota in response to DEX in intestinal I/R injury.
In the current study, the microbiota alteration and inflammatory response of mice undergoing intestinal I/R injury to DEX administration were determined. Treatments of DEX in combination with antibiotic treatment and fecal microbiota transplantation (FMT) were performed in a mouse model of intestinal I/R injury to investigate the protective role of microbiota. We hypothesized that DEX can modulate the microbiota abnormalities induced by intestinal I/R and thus prevent its hazardous effects on inflammatory factors, thus improving the mouse survival rate. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-824/rc).
Methods
Construction of an animal model of intestinal I/R injury
Experiments were performed under a project license (No. NFYY-2019-1037) granted by Committee of Southern Medical University, Nanfang Hospital, in compliance with Southern Medical University guidelines for the care and use of animals approved by the Institutional Animal Institutional Animal Care and Use Committee of Southern Medical University, Nanfang Hospital (Guangzhou, China). Six to eight-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Southern Medical University (Guangzhou, China). All mice were housed in a light-, temperature-, and humidity-controlled room with food and water available ad libitum. Intestinal I/R injury was induced by surgery according to the procedure described by Gubernatorova et al. (20). Mice were anesthetized with 1.5% isoflurane inhalation. The superior mesenteric artery (SMA) was exposed through a midline abdominal incision and completely clamped for 1 hour. This was followed by reperfusion for 3 hours as determined by Wang’s study which reported that mice undergoing reperfusion for 3 hours show significant change in bacterial composition (21). In the sham-operated group, the SMA was carefully dissected without occlusion. Lidocaine cream was used to reduce the pain of surgical incision, and mice were kept warm with an electric blanket. During the model construction, the experimenters were blinded to the treatment of the mice.
Experimental protocols
The animals were marked and randomly allocated to different groups by using random number generators. Three experiments were conducted with 10 mice per group. Control mice were injected with normal saline (NS) and/or underwent sham operation. No mice died within 3 hours of intestinal I/R. DEX or NS was injected intraperitoneally 24 hours before establishing I/R. During the study period, mice were kept sedated, body temperature was maintained at 37 ℃ with a heating pad, and mice were injected subcutaneously with 0.5 mL of NS for fluid resuscitation and returned to their cages immediately after reperfusion (Figure 1).
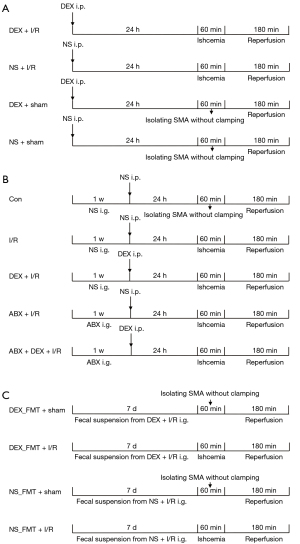
In experiment 1, mice were randomly divided into 4 groups which were treated as follows: in the (I) DEX + I/R group and (II) NS + I/R group, DEX or an equal volume of NS (400 µg/kg) was injected intraperitoneally, and mice were subjected to intestinal I/R injury; meanwhile, in the (III) DEX + sham operation group (DEX + sham) and (IV) NS + sham operation group (NS + sham), sham-operated mice were given DEX or an equal volume of NS. The dose of DEX was administered according to the protocol outlined by Zhang et al. (22). The mice underwent cervical dislocation, and the intestines were carefully exposed with forceps and scissors. Fecal samples were collected directly from the distal small intestine in tubes, as described by Jimeno et al. (23).
In experiment 2, mice were randomly divided into 5 groups. Four of the groups comprised mice treated with NS (I/R group), antibiotics (ABX + I/R group), DEX (DEX + I/R group), or a combination of antibiotics and DEX (ABX + DEX + I/R group) before intestinal reperfusion was induced. The control group (Con) consisted of sham-operated mice treated with NS. The antibiotic cocktail was based on that described in the study by Jimeno et al. (23), in which the administration of 200 µL ampicillin, gentamicin, neomycin, and vancomycin (all at 100 mg/mL) and metronidazole (10 mg/day) daily for 1 week to mice effectively decreased fecal bacteria without causing dehydration or weight loss.
In experiment 3, or the FMT study, fresh fecal pellets were collected from intestinal I/R mice receiving DEX or NS and dispersed in sterilized phosphate-buffered saline (PBS). The supernatant was used for transplantation in a manner reported by Bai et al. (24). Mice were randomly assigned to 4 groups, which received the following treatments: in the (I) DEX_FMT + sham group and (II) the DEX_FMT + I/R group, mice were orally administered a fecal suspension from DEX-preconditioned I/R mice and were subsequently subjected to a sham operation or intestinal I/R injury, respectively; meanwhile; in the (III) NS_FMT + sham group and (IV) the NS_FMT + I/R group, mice were subjected to oral gavage with fecal suspension from the I/R mice with NS pretreatment and were subsequently subjected to a sham operation or intestinal I/R injury, respectively.
Histopathological analysis
The distal ileum of the small intestine (3 cm proximal to the ileocecal valve) of each mouse was collected and washed with cold PBS buffer, then fixed in 4% paraformaldehyde solution, and embedded in paraffin. The tissues were stained with hematoxylin and eosin (HE). The pathological scores of the intestinal mucosal injuries were evaluated by randomly choosing 6 fields of intestine tissue according to the modified Chiu scoring system (25), and the average scores were used to determine mucosal damage. The technicians were blinded to the mice’s treatments.
Biochemical analysis of intestinal tissues and plasma
The intestinal tissues were rinsed and homogenized with precooled PBS. The homogenates were centrifuged at 4,000 ×g for 15 minutes, and the supernatants were used for further analysis. Mouse blood samples were collected using cardiac puncture and centrifuged at 1,000 ×g for 15 minutes. Plasma was prepared for biochemical analysis. Superoxide dismutase (SOD) activity (U/mg protein) was measured using a microplate reader, as previously described (26).
Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR)
The cecum contents were extracted into sterile dressings and stored in microcentrifuge tubes. Bacterial DNA was isolated from fecal samples according to a sodium dodecyl sulphate (SDS) modified gentle lysis-based protocol described by Bonot et al. (27). TRIzol Reagent (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was used to extract RNA from frozen intestinal tissue, and a Prime Script RT Reagent Kit (Takara Bio, Kusatsu, Japan) was used to reverse-transcribe RNA into complement DNA (cDNA). qRT-PCR was performed by mixing the SYBR Premix Ex Taq II kit (Takara) and cDNA in a PCR microarray plate. The fold changes in the target genes were calculated according to the 2−ΔΔCT method (28). The qRT-PCR primers were as follows: 16S, F: ACTCCTCCGGGAGGCAGCA, R: GGACTACHVGGGTWTCTCTAAT; Bacteroidetes, F: GGCGACCGGCGCACGGG, R: GRCCTTCCTCTCAGAACCC; Firmicutes, F: GGAGYATGTGGTTTAATTCGA, R: AGCTGACGACAACCATGCAC; 18S, F: AGAGTTTGATCCTGGCTCAG, R: TGCTGCCTCCCGTAGGAGTC; interleukin (IL)-6, F: CACATGTTCTCTGGGAAATCG, R: TTGTATCTCTGGAAGTTTCAGATTGTT; IL-1β, F: ACCTTCCAGGATGAGGACATGA, R: CTAATGGGAACGTCACACACCA; and tumor necrosis factor (TNF)-α, F: GCCACCACGCTCTTCTGTCTAC, R: GGGTCTGGGCCATAGAACTGAT.
Western blot analysis
Intestinal samples were collected in polypropylene tubes and lysed in RIPA buffer (Solarbio, Beijing, China), and the supernatants from the homogenates were collected after centrifugation. Total proteins were separated using sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Polyvinylidene fluoride (PVDF) membranes were blocked in Tris-buffered saline with Tween (TBST) buffer containing 5% nonfat milk at room temperature for 1 hour and incubated with primary antibodies overnight at 4 ℃ as follows: rabbit polyclonal antibody against occludin (Abcam, Cambridge, UK) at 1:500 and ZO-1 (Abcam) at 1:2,000 and horseradish peroxidase-conjugated secondary antibodies (Abcam) at 1:20,000.
Enzyme-linked immunosorbent assay (ELISA) analysis
IL-6 and C-reactive protein (CRP) levels were determined using commercial ELISA kits (Beyotime Technology, Shanghai, China), according to the manufacturer’s protocols. Cytokine levels in the samples were defined using a standard curve.
Survival rate
Mice in all groups were observed at 6, 12, 24, 48, and 72 hours after implementation of the intestinal I/R injury model.
Statistical analysis
All data analyses were performed with GraphPad Prism version 7.0 (GraphPad Software, Inc., San Diego, CA, USA), and investigators were blinded to the group allocations. All values in each group were graphed as mean and standard error of the mean (SEM) and were then analyzed with factorial analysis of variance (ANOVA), which was followed by Tukey’s post hoc test for comparison of multiple groups. The log-rank test was performed for the statistical analysis of survival. The expressions of IL-6, IL-1β, TNF-α, and CRP are presented as a fold change in the control group. Statistical significance was defined as a P value <0.05, and multiple comparisons were adjusted using a Bonferroni correction. According to a study on microbiota alteration in mice undergoing intestinal I/R, animal experimentation with ≥6 animals can eliminate individual differences (21). In addition, since the statistical analysis requires ≥3 data points, considering that the differences between animals could have been large, we included 10 mice in each group based on the “resource equation” method (29).
Results
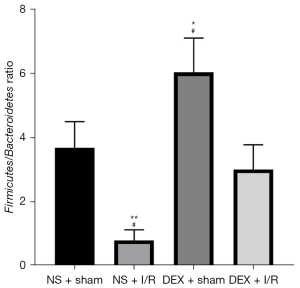
DEX reversed I/R-induced bacterial abnormalities
The dominant bacteria in the intestinal tract are Firmicutes and Bacteroidetes (30). Figure 2 shows changes in the relative gut microbiota composition, where Firmicutes and Bacteroidetes were increased after DEX administration in the NS group that was challenged with sham surgery (DEX + sham 6.05±0.51 vs. NS + sham 3.70±0.38; 95% CI: 0.95–3.75; P<0.05). Additionally, intestinal I/R injury reduced the ratio of Firmicutes to Bacteroidetes (NS + sham 3.70±0.38 vs. NS + I/R 0.82±0.15; 95% CI: 1.47–4.28; P<0.01), with the relative abundance of Bacteroidetes being increased (data not shown). However, DEX pretreatment reversed the abnormality of the Firmicutes to Bacteroidetes ratio induced by intestinal I/R (DEX + I/R 3.02±0.36 vs. NS + I/R 0.82±0.15; 95% CI: 0.80–3.60; P<0.05).
DEX prevented intestinal I/R injury in mice
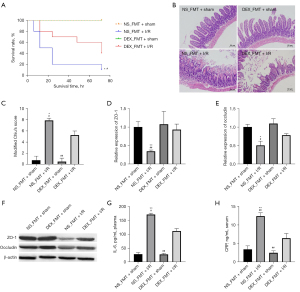
Using a rodent model of intestinal I/R injury, we recorded the living state of each mouse. We observed that all animals in the sham-operated group survived and that the mortality rates of both the DEX + sham and NS + sham groups were 0%. Thus, no significant difference in survival was detected between the sham-operated groups (Figure 3A). However, the mortality rates of the NS + I/R and DEX + I/R groups were 90% and 60%, respectively, and finally, 6 mice in the NS + I/R group and 4 mice in the DEX + I/R group died. The intestinal I/R-induced mortality was attenuated at 72 hours with a significant difference relative to the mice that were pretreated with DEX (NS + I/R 0.10±0.09 vs. DEX + I/R 0.40±0.16; P<0.05).
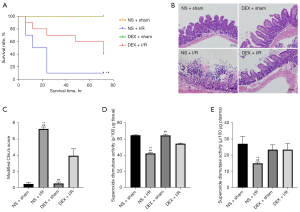
To evaluate mucosal injury after the induction of intestinal I/R injury, we examined histological changes in the intestinal tissue. Compared with sham-operated mice, mice subjected to 1 hour of intestinal ischemia and 3 hours of reperfusion showed significant mucosal damage, manifested by marked disintegrated epithelial villi and inflammatory cell infiltration. Figure 3B shows less extensive intestinal damage in the DEX + I/R group. Quantitatively, the morphological changes in the I/R groups, evaluated according to the modified Chiu scoring system, significantly decreased compared with those subjected to sham operations (NS + sham 0.45±0.11 vs. NS + I/R 7.25±0.24; 95% CI: 5.87–7.73; P<0.01); meanwhile, DEX attenuated the intestinal pathological damage induced by I/R (DEX + I/R 3.95±0.41 vs. NS + I/R 7.25±0.24; 95% CI: 2.37–4.32; P<0.01; Figure 3C).
To further evaluate the protective effect of DEX against intestinal I/R injury, we assessed and detected oxidative stress markers of intestinal I/R injury. We found that the SOD activity in intestinal tissue and plasma was significantly decreased in the I/R groups compared with the sham-operated groups (see Table S1). As expected, the anti-inflammation responses of the I/R group mice injected with DEX were significantly increased compared with those mice injected with NS (Figure 3D,3E).
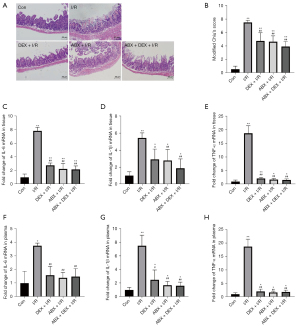
Effect of DEX on intestinal I/R injury after microbiota depletion by antibiotics
To identify the role of bacteria in mice with intestinal I/R injury, we observed intestinal changes following antibiotic administration. Compared to the control groups, the I/R groups showed more severe damage to the intestinal mucosa, and antibiotic administration resulted in the attenuation of histological damage. However, the difference in the protective effect against I/R injury was not significant between the ABX + DEX + I/R group and the ABX + I/R group, where lower levels of monocyte infiltration and milder epithelium blunting were detected (Figure 4A). Modified Chiu scores were consistent with the pathological changes in intestinal paraffin sections, and no significant difference was found between the ABX + I/R group and the ABX + DEX + I/R group (4.64±0.39 vs. 3.91±0.34; 95% CI: −0.80 to 2.25; P=0.66; Figure 4B). Previous studies report higher levels of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, present in intestinal I/R mice (31,32). Using qRT-PCR, we determined the messenger RNA (mRNA) expression of inflammatory cytokines after induction of intestinal I/R injury with or without antibiotic or DEX treatments. Figure 4C-4H shows a more severe intestinal injury induced by I/R via upregulation of IL-6, IL1-β, and TNF-α mRNA expression in intestinal tissue and plasma (see Table S2). These increases in inflammatory markers were significantly attenuated by antibiotics or administration of DEX alone. Nevertheless, cytokine expression was not further decreased by the concomitant use of antibiotics and DEX in intestinal tissue and plasma.
DEX-treated fecal transplants attenuated intestinal I/R injury
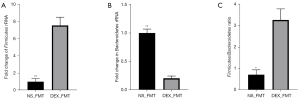
To further determine the role of the gut microbiota of DEX-treated mice in attenuating the intestinal injury of I/R mice, we transplanted feces from DEX-treated mice to intestinal I/R mice. As illustrated in Figure 5, the trend of Firmicutes/Bacteroidetes in feces in the DEX-treated group compared with the saline control group was consistent with that before fecal transplantation. The above results indicate that the FMT experiment was successful. All mice subjected to the sham-operated group survived (n=20), and thus the mortality rate was 0%. However, in mice that underwent intestinal I/R injury, the survival rate at 72 hours was significantly reduced from 100% to 10% compared to the sham-operated group, and 9 of 10 mice in the NS_FMT + I/R group and 6 of 10 mice in the DEX_FMT + I/R group died (Figure 6A). DEX-treated fecal transplants significantly increased the 72-hour survival of intestinal I/R mice. Figure 6B shows the pathological changes in intestinal tissue and illustrates how I/R damaged the intestinal mucosa, causing considerable epithelial cell shedding. To characterize the lesions induced by intestinal I/R, we assessed the modified Chiu scores, which were consistent with these findings, with the mean score being lower in mice that received feces from the DEX-treated groups vs. the NS control group (5.25±0.31 vs. 7.88±0.40; 95% CI: 1.36–3.89; P<0.05; Figure 6C). To characterize the lesions induced by intestinal I/R, we assessed the expression of ZO-1 and occludin, which are indicators of damage to the intestinal mechanical barrier. Figure 6D-6F show that the DEX-treated fecal transplants provided intestinal protection in mice subjected to intestinal I/R injury, as evidenced by the increased ZO-1 and occludin expression in the intestinal tissue (see Table S3).
We further examined the expression of inflammatory proteins. Markedly elevated IL-6 and CRP levels were detected following intestinal I/R. However, the production of IL-6 and CRP was inhibited by FMT from DEX-treated mice (see Table S3; Figure 6G,6H).
Discussion
The results of this study indicate an association between gut microbiota and DEX treatment in intestinal I/R injury. First, we found that DEX reversed I/R-induced bacterial abnormalities by increasing the ratio of Firmicutes to Bacteroidetes, which was accompanied by a decrease in the abundance of inflammatory cytokines and an improvement in survival rate in I/R mice. In addition, to elucidate the association between microbiota and the protective effect of DEX against intestinal I/R injury, mice were treated with an antibiotic cocktail to deplete gut microbiota. We found that the combination treatment of DEX and antibiotics showed no further protective effect against intestinal I/R injury compared to antibiotic treatment alone. Moreover, fecal transplants from DEX-treated mice inhibited the critical effects of I/R. Notably, the protective effect of DEX on intestinal I/R injury might be due to its effect on the modulation of the microbiota.
We further demonstrated that I/R caused severe intestinal pathological damage, which was contrary to the results of Grootjans et al.’s study, in which the mucosal integrity rapidly recovered within 120 minutes of reperfusion in animals and humans (33). However, in our study, mice were exposed to 60 minutes of ischemia with 180 minutes of reperfusion, and other research indicates that the sensitivity to ischemia varies in different parts of the intestine (34). Wang et al. found that severe necroses with hemorrhage of colon mucosa could be detected after 3 hours of reperfusion and tended to recover at 6 hours of reperfusion (21). Moreover, the structure of blood supply in mice, rats, and human beings is variable (34), and this may be the reason for the discrepancies in the results.
Several studies have demonstrated the existence of microbiota alterations in intestinal I/R injury (11,35). In this study, we found that DEX increased the relative quantity of Firmicutes and the Firmicutes to Bacteroidetes ratio, further reversing the intestinal I/R-induced microbiota abnormalities. Firmicutes and Bacteroidetes are 2 major phyla in normal intestinal microbiota, and the Firmicutes to Bacteroidetes ratio has been associated with several pathological conditions (36). Huang et al. found that a high-fiber diet increased the quantity of Firmicutes and reduced the incidence of rectal cancer (37). However, contradictory results indicate that a high-fat diet increased the expression of Firmicutes, suggesting that this was related to inflammation (38). This evidence evinces the complexity of the impact of intestinal flora on health. More research is needed to reveal the roles of Firmicutes and Bacteroidetes in different models. The ratio of Firmicutes to Bacteroidetes may be useful information in early disease diagnosis and prognosis.
We also sought to investigate whether the protective effect of DEX was microbiota-dependent, and an antibiotic protocol was employed to construct pseudo-germ-free mice. In this study, treatment with antibiotics attenuated intestinal I/R damage, which is consistent with the findings of Yoshiya et al. (12). However, in another study, antibiotics could not reduce intestinal injury or distant organ failure caused by intestinal I/R (13). This may be related to the method selected to create the intestinal I/R model and the various times of artery occlusion and declamping. Evidence suggests that the severity of I/R injury is dependent on the duration of ischemia. Nevertheless, we also found that DEX did not further improve intestinal I/R injury in antibiotic-treated mice, indicating that the protective effect of DEX was based on the intestinal microbiota. However, this may be a nonbiological reaction caused by the combined use of DEX and antibiotics, which suggested that antibiotics inhibited the protective effect of DEX. Therefore, FMT was introduced to reveal the mechanism by which DEX improves intestinal I/R injury. We found that feces collected from DEX-treated animals could alleviate intestinal injury by upregulating tight junction proteins and reducing the levels of inflammation factors.
In animal experiments, DEX has been shown to inhibit inflammation. We found that inflammatory cytokines were significantly upregulated in intestinal I/R mice and that DEX treatment could revert these intestinal abnormalities. A previous study has demonstrated the association between commensals and pathogens. Commensals have been shown to promote immunity development, limit pathogen growth, and prevent inflammation-induced damage to the host (12). Thus, the restoration of the gut microbiota induced by DEX suppresses inflammation and attenuates intestinal damage in mice.
It is known that I/R injury often occurs in surgeries involving the intervention of abdominal organs and blood vessels, resulting in life-threatening complications. Recent therapeutic interventions have thus focused on oxidant injury and neutrophil activation. However, the efficacy of these interventions remains unclear, which limits their clinical utility (37). Several studies have found microbiota to be associated with the development of I/R injury (12,39). DEX is a commonly used sedative in anesthesia, and our study investigated its role in modulating microbiota; the subsequent results have clarified understand the correlation between intestinal I/R and microbiota, which may have potential therapeutic value.
Although the translation of animal study findings into clinical applications may be inefficient, such translation plays a positive role in alleviating suffering and improving patient outcomes. Our study simulated the process of intestinal ischemia and reperfusion during surgery. Due the progress in the medical sciences, the importance of anesthesiologists is expanding in perioperational management. The discovery of DEX’s protective effect on microbiota may yet bring benefit to patients. In addition, other studies have found that similar preintestinal I/R approaches to modulating microbiota, including the use of probiotic bacteria and gut decontamination, may become viable treatments in clinical practice (40,41).
There are several limitations to this study. First, we focused on the injury occurring in the intestine and not that in distant organs. Second, although we found that DEX affected the intestinal microbiota and improved intestinal I/R injury by altering the ratio of Firmicutes to Bacteroidetes, the exact mechanism by which it affects the intestinal microbiota remains unclear. In addition, the degree of improvement of intestinal I/R injury caused by the alterations of the intestinal microbiota warrants further investigation. The restoration of intestinal mucosal integrity is another limitation of this study, which we will further explore in future studies. Finally, we only examined the relative expression of Firmicutes and Bacteroidetes, and including other specific intestinal flora may provide insight into the mechanisms by which DEX attenuates intestinal I/R injury.
Conclusions
In conclusion, this study demonstrated that DEX can alleviate intestinal I/R injury in mice, which may be attributed to the regulation of the intestinal microbiota and the inhibition of inflammation. Our findings suggest that determining the Firmicutes to Bacteroidetes ratio may provide opportunities for diagnostic tests and assessment of the severity of intestinal I/R injury. It further follows that restoring balance in the intestinal microbiota may be a potential treatment for patients with I/R injury. In addition, DEX may be beneficial for patients with severe dysbiosis owing to its effect on microbiota modulation. The findings of this study provide a new understanding of the mechanism of DEX and its potential use in the management of intestinal I/R injury.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant No. 81730058 and No. 82172141 to KXL; grant No. 82902010 to JJH).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-824/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-824/dss
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-824/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-824/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. NFYY-2019-1037) granted by Committee of Southern Medical University, Nanfang Hospital, in compliance with Southern Medical University guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yu H, Kirkpatrick IDC. An Update on Acute Mesenteric Ischemia. Can Assoc Radiol J 2022; Epub ahead of print. [Crossref] [PubMed]
- Corcos O, Nuzzo A. Gastro-intestinal vascular emergencies. Best Pract Res Clin Gastroenterol 2013;27:709-25. [Crossref] [PubMed]
- Li G, Wang S, Fan Z. Oxidative Stress in Intestinal Ischemia-Reperfusion. Front Med (Lausanne) 2021;8:750731. [Crossref] [PubMed]
- Tassopoulos A, Chalkias A, Papalois A, et al. The effect of antioxidant supplementation on bacterial translocation after intestinal ischemia and reperfusion. Redox Rep 2017;22:1-9. [Crossref] [PubMed]
- Liao S, Luo J, Kadier T, et al. Mitochondrial DNA Release Contributes to Intestinal Ischemia/Reperfusion Injury. Front Pharmacol 2022;13:854994. [Crossref] [PubMed]
- Mi L, Zhang N, Wan J, et al. Remote ischemic post-conditioning alleviates ischemia/reperfusion-induced intestinal injury via the ERK signaling pathway-mediated RAGE/HMGB axis. Mol Med Rep 2021;24:773. [Crossref] [PubMed]
- Manos J. The human microbiome in disease and pathology. APMIS 2022; Epub ahead of print. [Crossref] [PubMed]
- Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res 2017;120:1183-96. [Crossref] [PubMed]
- Ejtahed HS, Hasani-Ranjbar S, Larijani B. Human Microbiome as an Approach to Personalized Medicine. Altern Ther Health Med 2017;23:8-9. [PubMed]
- Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017;5:e1373208. [Crossref] [PubMed]
- Wang F, Li Q, Wang C, et al. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PloS One 2012;7:e42027. [Crossref] [PubMed]
- Yoshiya K, Lapchak PH, Thai TH, et al. Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol 2011;301:G1020-30. [Crossref] [PubMed]
- Souza DG, Vieira AT, Soares AC, et al. The essential role of the intestinal microbiota in facilitating acute inflammatory responses. J Immunol 2004;173:4137-46. [Crossref] [PubMed]
- Kumar KR, Saeed Z, Chhabra A. Optimal utilization of sedative and analgesic potential of dexmedetomidine in a child with severe kyphoscoliosis for vitreoretinal surgery. J Anaesthesiol Clin Pharmacol 2020;36:427-8. [Crossref] [PubMed]
- Liu X, Li Y, Kang L, et al. Recent Advances in the Clinical Value and Potential of Dexmedetomidine. J Inflamm Res 2021;14:7507-27. [Crossref] [PubMed]
- Zhang XY, Liu ZM, Wen SH, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology 2012;116:1035-46. [Crossref] [PubMed]
- Shen J, Fu G, Jiang L, et al. Effect of dexmedetomidine pretreatment on lung injury following intestinal ischemia-reperfusion. Exp Ther Med 2013;6:1359-64. [Crossref] [PubMed]
- Fan X, Du J, Wang MH, et al. Irisin Contributes to the Hepatoprotection of Dexmedetomidine during Intestinal Ischemia/Reperfusion. Oxid Med Cell Longev 2019;2019:7857082. [Crossref] [PubMed]
- Chen Y, Bian W, Xu B. Pretreatment with dexmedetomidine alleviates lung injury in a rat model of intestinal ischemia reperfusion. Mol Med Rep 2020;21:1233-41. [Crossref] [PubMed]
- Gubernatorova EO, Perez-Chanona E, Koroleva EP, et al. Murine Model of Intestinal Ischemia-reperfusion Injury. J Vis Exp 2016;53881. [PubMed]
- Wang F, Li Q, He Q, et al. Temporal variations of the ileal microbiota in intestinal ischemia and reperfusion. Shock 2013;39:96-103. [Crossref] [PubMed]
- Zhang Z, Ferretti V, Güntan İ, et al. Neuronal ensembles sufficient for recovery sleep and the sedative actions of α2 adrenergic agonists. Nat Neurosci 2015;18:553-61. [Crossref] [PubMed]
- Jimeno R, Brailey PM, Barral P. Quantitative Polymerase Chain Reaction-based Analyses of Murine Intestinal Microbiota After Oral Antibiotic Treatment. J Vis Exp 2018; [Crossref] [PubMed]
- Bai T, Zhang L, Wang H, et al. Fecal Microbiota Transplantation Is Effective in Relieving Visceral Hypersensitivity in a Postinfectious Model. Biomed Res Int 2018;2018:3860743. [Crossref] [PubMed]
- Hu J, Deng F, Zhao B, et al. Lactobacillus murinus alleviate intestinal ischemia/reperfusion injury through promoting the release of interleukin-10 from M2 macrophages via Toll-like receptor 2 signaling. Microbiome 2022;10:38. [Crossref] [PubMed]
- Liu KX, Rinne T, He W, et al. Propofol attenuates intestinal mucosa injury induced by intestinal ischemia-reperfusion in the rat. Can J Anaesth 2007;54:366-74. [Crossref] [PubMed]
- Bonot S, Courtois S, Block JC, et al. Improving the recovery of Qpcr-grade DNA from sludge and sediment. Appl Microbiol Biotechnol 2010;87:2303-11. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother 2013;4:303-6. [Crossref] [PubMed]
- Valitutti F, Cucchiara S, Fasano A. Celiac Disease and the Microbiome. Nutrients 2019;11:2403. [Crossref] [PubMed]
- Wu H, Deng YY, Liu L, et al. Intestinal ischemia-reperfusion of macaques triggers a strong innate immune response. World J Gastroenterol 2014;20:15327-34. [Crossref] [PubMed]
- Zeng Z, Liu HM, Zhang YY, et al. Aggravated intestinal ischemia reperfusion injury is associated with activated mitochondrial autophagy in a mouse model of diabetes. Mol Med Rep 2020;22:1892-900. [Crossref] [PubMed]
- Grootjans J, Thuijls G, Derikx JP, et al. Rapid lamina propria retraction and zipper-like constriction of the epithelium preserves the epithelial lining in human small intestine exposed to ischaemia-reperfusion. J Pathol 2011;224:411-9. [Crossref] [PubMed]
- Gonzalez LM, Moeser AJ, Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol 2015;308:G63-75. [Crossref] [PubMed]
- Zhang XY, Liu ZM, Zhang HF, et al. TGF-β1 improves mucosal IgA dysfunction and dysbiosis following intestinal ischaemia-reperfusion in mice. J Cell Mol Med 2016;20:1014-23. [Crossref] [PubMed]
- Di Pierro F. Gut Microbiota Parameters Potentially Useful in Clinical Perspective. Microorganisms 2021;9:2402. [Crossref] [PubMed]
- Huang P, Liu Y. A Reasonable Diet Promotes Balance of Intestinal Microbiota: Prevention of Precolorectal Cancer. Biomed Res Int 2019;2019:3405278. [Crossref] [PubMed]
- Wan Y, Wang F, Yuan J, et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: a 6-month randomised controlled-feeding trial. Gut 2019;68:1417-29. [Crossref] [PubMed]
- Kalogeris T, Baines CP, Krenz M, et al. Ischemia/Reperfusion. Compr Physiol 2016;7:113-70. [Crossref] [PubMed]
- Rizwan R, Feuerstadt P. Bad blood: ischemic conditions of the large bowel. Curr Opin Gastroenterol 2022;38:72-9. [Crossref] [PubMed]
- Jung CY, Bae JM. Pathophysiology and protective approaches of gut injury in critical illness. Yeungnam Univ J Med 2021;38:27-33. [Crossref] [PubMed]


