
Retrospective comparative cohort study of neovagina creation by modified Vecchietti-laparoendoscopic single-site surgery for Mayer-Rokitansky-Küster-Hauser syndrome
Introduction
Mayer-Rokitansky-Küster-Hauser syndrome (MRKHS), or congenital Müllerian agenesis, is characterized by a distortion of the female vaginal duct in phenotypically normal women with a 46,XX karyotype (1). The incidence of MRKHS is 1 in 4,500–5,000 (2). Artificial vaginoplasty is indicated for patients with MRKHS who have failed parietal compression therapy or who have actively opted for surgical treatment (3). The history of vaginoplasty has evolved over 200 years. Trends in the management of vaginoplasty include simplification, minimal trauma, the use of autologous tissue to reconstruct the vagina, and the use of laparoscopic techniques instead of traditional open surgery. There is no consensus regarding the optimal surgical technique (4). The ideal artificial vagina must have the characteristics of a normal female vagina, i.e., sufficient length, width, elasticity, a suitable vaginal axis, secretion and lubrication functions, and fully meet the physiological and psychological needs of patients. Second, the ideal artificial vagina must be minimally traumatic or even noninvasive to obtain maximum benefits. Thus, our goal was to create or rebuild an artificial vagina with physiological function that did not harm other physiological functions of the patient and did not require special care after surgery.
Currently, a variety of surgical methods for neovagina formation have been reported and analyzed, such as lining the cavity with various types of materials after dissecting the space between the rectum and bladder, including covering the autogenous skin graft (McIndoe procedure) (5), using peritoneum from the pouch of Douglas (Davydov procedure) (6,7), or performing bowel vaginoplasty with a rectosigmoid segment (8). Vecchietti vaginoplasty is a surgical procedure that meets all of the above needs. The length of the artificial vagina after a Vecchietti vaginoplasty averages 8–12 cm. The Vecchietti vaginoplasty is most often performed laparoscopically. Previously, Vecchietti vaginoplasty was performed under the multiport laparoscope. Although traditional multi-hole laparoscopic surgery is minimally invasive, it can still leave surgical scars on the lower abdomen, which cannot meet the aesthetic requirements of many young women.
After generations of continuous improvement, the technology underlying the laparoscopic Vecchietti vaginoplasty has matured, especially given the recent combination of single-site laparoscopic technology and Vecchietti vaginoplasty. The Vecchietti procedure, which was first introduced in 1965 (9), meets the above standards. Modern surgical procedures have increasingly adopted minimally invasive surgical techniques to achieve a satisfactory cosmetic effect. Less invasiveness, a superior cosmetic result, less pain, higher patient satisfaction, and faster recovery have all been touted as advantages of laparoendoscopic single-site surgery (LESS) over traditional laparoscopy (10). A novel approach of LESS-vaginoplasty was launched in our center in 2019. LESS-vaginoplasty has attracted considerable attention among both academic physicians and patients. The transumbilical incision has good concealment, and the surgical scar is covered by the navel. On the premise of ensuring the surgical effect, it meets the increasing aesthetic needs of women. Recently, it has been favored by women. But an approximate 2.5 cm incision is made in the navel for single-port laparoscopic surgery, the navel is the weakest part of the abdominal wall, and the umbilical incision carries the risk of incisional hernia, infection, and dehiscence. This study compared the effects of a modified Vecchietti-laparoendoscopic single-site surgery (MVLESS) with self-made instruments to the traditional multi-incision Vecchietti (TMV) on creating a neovagina for MRKHS patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4360/rc).
Methods
We conducted a retrospective comparative cohort study of the clinical data from MRKHS patients treated in the Department of Difficult Gynecological Disease at Xi’an People’s Hospital between January 2010 and December 2020. A total of 36 patients who were diagnosed with MRKHS and underwent a Vecchietti vaginoplasty (created by author Dr. Shuncang Zhang) were retrospectively analyzed. Study size was not predetermined. All eligible participants had congenital vaginal agenesis with a 46,XX karyotype and normal ovarian function. Of the patients who met the inclusion criteria, two study groups were formed. Women who underwent the modified MVLESS procedure to create a new vagina were designated as the study group (n=14). The patients who underwent the TMV procedure were designated as the control group (n=22). The patients were selected for investigating traits related to participants with congenital vaginal agenesis, having the largest sample sizes, and consisting of the most similar populations while minimising sample overlap. This study analyzed peri- and postoperative data and differences in clinical outcomes between the 2 groups, including the operative time, amount of intraoperative bleeding, blood transfusions administered, postoperative systemic inflammatory response syndrome, postoperative hospital stay, postoperative complications, and the Female Sexual Function Index (FSFI) (11) and Female Genital Self-Image Scale (FGSIS) (12) at the 12-month follow-up evaluation.
Selection of participants and criteria
In accordance with the American College of Obstetricians and Gynecologists committee opinion No. 728[3]. The inclusion criteria are outlined below. Women with congenital vaginal agenesis (no vagina or a vagina <3 cm in length), normal external genitalia and ovarian function, and absence of the uterus or fibrous remnants were included in this study (3) (Figure 1). All of the patients were emotionally mature and had a stable sexual relationship and a strong desire to have a new vagina created. The duration of postoperative follow-up was 12 months. Adequate compliance and initiative for long-term follow-up management were also needed. Patients <18 years of age or patients with extensive pelvic adhesions or any cancer were not included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics Board of Xi’an People’s Hospital (Xi’an Fourth Hospital) (No. 20220117), and informed consent was taken from all the patients.
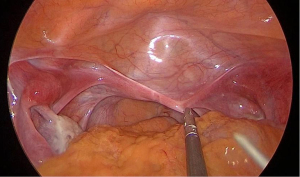
Preoperative preparation of patients and instrumentation
Each patient had a normal 46,XX karyotype and underwent a B-scan ultrasound and magnetic resonance imaging (MRI) of the pelvis and urinary tract to screen for concomitant genitourinary malformations. In addition, the hormonal profile was assessed prior to the procedure. A simplified traction device consisted of two specially-designed needles, two 50-cm 10-0 silk wires, an acrylic button, and a rubber pad with a diameter of 2.5 cm. Different sizes of glass vaginal prostheses and rolls of gauze were prepared. A No. 1 stainless-steel puncture needle was fashioned de novo with a diameter of 5 mm and 50 cm in length. The needle tip had a 1-cm pinhole and the other end was hemispherical (Figure 2). The hemispherical end of the needle was used to divide the cysto-rectal space, and the narrow-tapered end was used for puncturing and passing the traction wire. The No. 1 needle is patented under Chinese national patent protection (patent number: ZL200520002194.5). A No. 2 stainless-steel puncture needle was fashioned de novo with a diameter of 2 mm and 15 cm in length (Figure 3). The handle was designed to be held like a writing instrument; the length was 5 cm, the diameter was 5 mm, and the needle hole was approximately 5 mm from the tip. The button and rubber pad were sewn together with a 10-gauge silk thread that connected the button to 1 surface of the rubber pad. The rubber pad was placed in close contact with the vestibular mucosa, leaving approximately 40 cm of silk thread on 1 side of the rubber pad (soaked in 0.5% iodophor solution for 8 hours before surgery).
Procedures
The MVLESS procedure was performed under general anesthesia. The abdominal skin and vulva were routinely disinfected with iodophor, and a catheter was inserted into the urethra laparoscopically. A 15-mm longitudinal incision was made at the umbilicus and 3 trocars were inserted into the abdominal cavity via the fascial layer (Figure 4). The CO2 insufflation pressure was maintained at 12–15 mmHg during the procedure. The subsequent key steps of our new technique were as follows: under laparoscopic view, the vesicorectal peritoneal folds of the pelvic floor were incised with a monopolar coagulator to a length of 2.5–3 cm, and full blunt dilation of the vesicorectal and vesicovaginal spaces was performed with the hemispherical end of a No. 1 needle. The No. 1 needle or laparoscopic aspiration device was used to bluntly dilate to the level of the vestibular mucosa. Abdominal access to the No. 1 needle was obtained through a 5-mm trocar, and the cysto-rectal space was then separated. The surgeon then passed the tip of the needle through the vestibular mucosa at the 3 and 9 o’clock positions, with a distance of 10–15 mm between the 2 points. Two silk threads were introduced into the peritoneal cavity through the vesicorectal space by passing each of the 2 threads through the needle hole of the No. 1 needle. The vesicorectal peritoneum of the pelvic floor was then closed with a 1-0 absorbable interrupted suture.
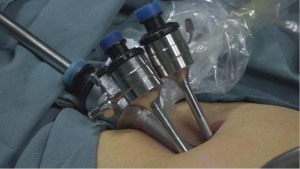
A No. 2 needle was inserted into the abdominal cavity 3 cm above the pubic symphysis at approximately 1 cm adjacent to the midline of the abdomen (2-cm distance between the 2 puncture points). The tapered end was inserted into the pelvis, and the conical end was fixed outside the body. The silk thread in the pelvic cavity was threaded into the needle hole. The vestibular mucosa was lifted at the 3 and 9 o’clock positions from the anterior abdominal wall, fixed on a rolled gauze-fixator, and tied into a bow (Figure 5).
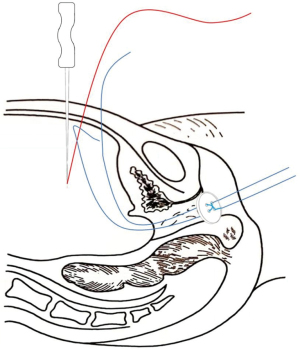
The traction line was elevated by 0.5–1 cm daily at the same time, and the degree of button elevation was adjusted according to the tightness of the traction line to avoid rupture of the vestibular mucosa. When the depth of the artificial vagina was approximately 4 cm, a glass mold was used as a top compression reinforcement to increase the force on the button, thus helping to increase the width and depth of the artificial vagina.
The TMV procedure was performed under general anesthesia. The patient was placed in a dorsal lithotomy position. The 2.5–3 cm transverse incision of the pelvic floor peritoneum was made with an electrocoagulation needle from the peritoneal fold between the 2 muscles, and the hemispherical end of the puncture lead needle or a flushing suction device was used to separate the tissue of the urinary cysto-rectal space to the level of the vestibular mucosa under water pressure. The 2 puncture points were 1–1.5 cm apart, and 2 silk threads were passed through the puncture eyes separately. The 2 silk threads were brought into the abdominal cavity through the urethral cysto-rectal space, and the pelvic floor peritoneum was sutured with 1–2 stitches. The 2 traction wires were lifted through the puncture device and led from the anterior abdominal wall with a rubber pad against the vestibular mucosa. The 2 traction wires were lifted from the abdomen through a rubber tube approximately 5 cm in length with a hole in the middle and fixed to the anterior abdominal wall. The traction wires were raised daily, and the degree of button rise was adjusted according to the tension (0.5–1 cm daily) so as not to rupture the vestibular mucosa. When the artificial vagina was approximately 4 cm deep, a gauze model was added to maintain the width of the artificial vagina. A glass model was used when the artificial vagina was >5 cm deep, fixed with fixation tape, and changed daily.
Postoperative management
A postoperative urethral indwelling catheter was left in place for 6–7 days until the button and traction cord were removed. Sexually-active women were cleared to resume sexual activity 4 weeks after the use of the vaginal mold. Women who had not had a sexual debut were cleared for sexual activity 3 months after the use of the vaginal mold. Patients completed the FSFI and FGSIS at the 12-month follow-up evaluation.
Follow-up and outcomes follow-up procedures were established for each participant. Clinical follow-up at 1, 3, 6 and 12 months after surgery and every 6 months thereafter. Mean follow-up duration was 28 months (13−62 months). During each visit, the neovaginal length and width, the epithelium of the vaginal mucosa was measured by colposcopy and Schiller’s test. At the 12-month postoperative follow-up, both the patients in the study group and control group required completion of the FSFI and FGSIS questionnaire. The effects of our procedure, including the anatomic efficacy (the length of the neovagina was at least 8 cm within 6 months of the surgery) and the functional efficacy (patients obtained a satisfactory sexual life 6 months after vaginoplasty), were the primary outcomes. The perioperative complications and long-term postoperative discomfort such as vaginal discharge, dyspareunia, and pelvic organ prolapse were reviewed and recorded as the secondary outcomes.
Statistical analysis
All data were analyzed using SPSS 19.0. The results are expressed as the mean ± standard deviation (SD) or percentage. The age distributions were similar in cases and controls because of matching. Time intervals were tested by one-way analysis of variance (ANOVA). Other data were qualitatively analyzed using a chi-square test and quantitative variables were analyzed using a Student t-test. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Clinical characteristics of the study population
A total of 36 Han Chinese patients with MRKHS underwent laparoscopic Vecchietti vaginoplasty [MVLESS (study group), n=14; and TMV (control group), n=22].
In the study group, 3 patients (21.43%) were sexually active. The mean age of these patients was 26.72±4.56 years (range, 21–32 years) and the mean body mass index was 21.06±1.79 kg/m2. All patients were diagnosed with type I MRKHS. In the control group, 6 patients (27.27%) were sexually active. The mean age of these patients was 25.86±3.97 years (range, 20–29 years), and the mean body mass index was 20.96±1.82 kg/m2. Twenty-one patients were diagnosed with type I MRKHS (95.45%), while the remaining patient (4.55%) had type II MRKHS, i.e., no left kidney. The clinical characteristics of the MRKHS patients are shown in Table 1.
Table 1
| Characteristic | Study group | Control group | P |
|---|---|---|---|
| Total number of patients with MRKHS | 14 | 22 | 0.84 |
| Mean age at vaginoplasty [min–max] (y) | 26.72±4.56 [21–32] | 25.86±3.97 [20–29] | 0.98 |
| Body mass index (kg/m2) | 21.06±1.79 | 20.96±1.82 | 0.75 |
| Sexual contact history | 3 (21.43) | 6 (27.27) | 0.86 |
| Length of vaginal pouch or cecum (mm), min–max | 0–4 | 0–4 | 0.85 |
| Patients with Mullerian fibrotic remnants | 9 (64.29) | 18 (81.82) | 0.82 |
| Type I MRKHS | 14 (100.00) | 21 (95.45) | 0.63 |
| Type II MRKHS | 0 | 1 (4.55) | 0.65 |
| Concomitant renal malformations | 0 | 1 (4.55) | 0.54 |
| Heterotopic ovary/ovaries | 0 | 0 | – |
| Congenital scoliosis | 0 | 0 | – |
| Unilateral or bilateral congenital hearing loss | 0 | 0 | – |
| Associated cardiovascular abnormalities | 0 | 0 | – |
| Longing neovagina duration (y), min–max | 1–3 | 3–10 | 0.81 |
Data are presented as the mean ± SD and number (%). MRKHS, Mayer-Rokitansky-Küster-Hauser syndrome; min, minimum; max, maximum; SD, standard deviation.
Surgical outcomes of neovagina creation
All procedures in both groups were successfully completed without conversion to open surgery. In both groups, the neovagina was wide with good elasticity, softness, smoothness, and lubrication. There were no significant differences in operative time (37±6 vs. 39±4 minutes, P=0.98), amount of intraoperative bleeding (28.32±9.82 vs. 29.45±3.84 mL, P=0.86), postoperative anal exsufflation time (18±4 vs. 20±4 hours, P=0.82), and duration of postoperative hospital stay (7±2 vs. 8±2 days, P=0.84) between the MVLESS and TMV groups. No intraoperative complications or perioperative deaths occurred (Table 2).
Table 2
| Surgical outcomes | Study group | Control group | P |
|---|---|---|---|
| Operative time (min) | 37±6 | 39±4 | 0.98 |
| Intraoperative bleeding (mL) | 28.32±9.82 | 29.45±3.84 | 0.86 |
| Postoperative hospital stay (d) | 7±2 | 8±2 | 0.84 |
| Postoperative anal exsufflation time (hours) | 18±4 | 20±4 | 0.82 |
| Length of neovagina at discharge (cm) | 9.40±1.14 | 9.37±1.72 | 0.75 |
| Median VAS score during traction [min–max] | 5 [3–7] | 6 [3–8] | 0.78 |
| Perioperative complications | |||
| Bladder injury | 0 | 0 | – |
| Intestinal fistula or urethral necrosis | 0 | 3 (13.64) | 0.68 |
| Local or systemic infection | 1 (7.14) | 0 | 0.58 |
| Hematoma | 0 | 0 | – |
| Median duration of follow-up [min–max] (mo) | 24 [12–36] | 60 [36–120] | 0.42 |
| Long-term postoperative discomfort | |||
| Abnormal vaginal discharge | 0 | 0 | – |
| Deep dyspareunia | 1 (7.14) | 2 (9.09) | 0.47 |
| Vaginismus* | 0 | 1 (4.55) | – |
| Prolapse of pelvic organ | 0 | 0 | – |
| Vaginal atresia owing to readhesion | 0 | 0 | – |
Data are presented as the mean ± SD and number (%). *, vaginismus: muscular contraction that causes the vagina to close as an anxiety-related reaction to sexual intercourse. VAS, visual analog scale; min, minimum; max, maximum; SD, standard deviation.
One patient in the study group had a postoperative fever, but there were no signs of infection on the wound surface of the abdomen and vagina. Other than pain at the surgical site, the patient had no other complaints of discomfort. Routine blood tests showed a slight increase in the leukocyte count, and after 96 hours of intravenous antibiotic treatment, the body temperature returned to normal; thus, the presence of a latent occult infection could not be ruled out.
Three patients (13.64%) in the control group developed rectovaginal fistulas, which occurred on postoperative days 6, 7, and 9. The traction threads were immediately removed, and the fistulas healed spontaneously within 3 months without any surgical intervention. After the rectovaginal fistulas healed, the patients continued to use vaginal compression molds and all eventually achieved a satisfactory vaginal length. All patients in both groups suffered from pain.
Results of FSFI and FGSIS scores
Based on the 12-month postoperative follow-up data, the FSFI ratings in the study and control groups demonstrated that patients who had undergone upgraded laparoscopic Vecchietti surgery had an excellent quality of sexual life (Table 3); however, sexual function was significantly better in the study group than the control group [lubrication (4.72±0.86 vs. 4.64±1.01, P=0.023), visual analog scale (VAS; 4.26±0.52 vs. 4.45±0.39, P=0.041), total FSFI score (30.21±4.32 vs. 28.42±2.21, P=0.048), and FGSIS score (23.21±1.98 vs. 22.14±2.04, P=0.012)].
Table 3
| Variable | Study group | Control group | P |
|---|---|---|---|
| FSFI measure | |||
| Desire | 4.62±1.57 | 3.98±1.54 | 0.412 |
| Arousal | 4.74±1.21 | 4.21±0.98 | 0.213 |
| Lubrication | 4.72±0.86 | 4.64±1.01 | 0.023 |
| Orgasm | 5.72±0.82 | 5.03±0.69 | 0.269 |
| Satisfaction | 5.64±0.72 | 5.42±0.68 | 0.245 |
| VAS | 4.26±0.52 | 4.45±0.39 | 0.041 |
| Total FSFI scores | 30.21±4.32 | 28.42±2.21 | 0.048 |
| FGSIS score | 23.21±1.98 | 22.14±2.04 | 0.012 |
| Number of participants | 14 | 22 | – |
Time point of follow-up 12 months after surgery. SD, standard deviation; FSFI, Female Sexual Function Index; VAS, visual analog scale; FGSIS, Female Genital Self-Image Scale.
Discussion
Vaginoplasty
MRKHS is distinguished by normal secondary sexual character development and a normal female karyotype 46,XX but with congenital aplasia of the uterus and 2/3 of the superior regions of the upper vagina (13). For a long time, MRKHS was thought to be an isolated occurrence, but the literature on known cases supports the assumption that a specific genetic substratum exists, and the syndrome appears to be transmitted as a dominant autosomal character with penetrative incomplete capacity and variable expressivity (13). Amenorrhea is typically the initial symptom in phenotypically normal women with normal 46,XX karyotype, physiology, and ovarian architecture, as well as a lack of excess androgens (14). Differential diagnosis has been investigated in cases of primary amenorrhea with normal secondary sexual characteristics and gonadal dysgenesis. It is therefore critical to confirm primary amenorrhea, normal sexual secondary characteristics of the congenital loss of the uterus, and vaginal atresia. Müllerian aplasia is not indicated by a vaginal transverse septum or an imperforate hymen (15). Ultrasound is a valuable diagnostic technique for precisely defining pelvic anatomy (16-18). The first study to test the theory that freemartinism in humans causes MRKHS was conducted in 2019 (19). Genitourinary tract (especially uterine abnormalities), heart, and musculoskeletal system malformations have also been reported (20). Even if the patients have an adequate ovarian function and female psychosexual identity, vaginal agenesis or absence might severely impair sexual activity (21).
MVLESS
MVLESS-vaginoplasty is performed through a small incision in the umbilicus. TMV, in contrast, is performed using a traditional multiport laparoscopic approach or a single-port trocar (Octoport; Dalim, Seoul, South Korea). Considering the cost of single-port trocars, our center performs single-site laparoscopic surgery with 3 trocars inserted through the fascial layer, eliminating the need for other special equipment. Because there is only 1 small incision through which all laparoscopic instruments enter and exit, good bilateral arm cooperation is required. Our center has performed a large number of single-site laparoscopic tubal ligations, ovarian cystectomies, myomectomies, ovarian cyst removals, and other laparoscopic procedures. Continuous improvements and developments have shortened the time of these procedures (typically, 40 minutes), which in turn reduces the trauma to the patient and associated procedural risks.
Performing the needle puncture and pulling the intra-abdominal thread out of the abdominal cavity are key to the success of a MVLESS. By using the hemispherical end of a specially-designed No. 1 needle or a laparoscopic suction device under laparoscopic view, the vesicorectal and vesicovaginal spaces can be separated under hydraulic pressure, thus reducing intraoperative tissue damage without visible scars and facilitating a satisfactory postoperative sex experience. Vecchietti vaginoplasty with the aid of laparoscopy has received wide acceptance since 1994 (22). Procedures based on the Vecchietti approach tend to accomplish full epithelialization of the neovagina, and it has been established that the neovagina has finer sensitivity as a result (22).
There were no significant differences in operative time, postoperative anal exsufflation time, or length of postoperative hospital stay between the MVLESS and TMV groups. Based on the 12-month follow-up data, the FSFI and FGSIS scores indicated a high quality of sexual satisfaction in both groups; however, sexual function in the study group was much better than in the control group.
The benefits of TMV vaginoplasty are preserved in MVLESS and streamlined. Patients who underwent the novel procedure achieved 100% anatomic success and functional efficacy, which was consistent with prior research findings (23). Patients with vaginal agenesis also had near-normal sexual function and satisfactory sexual experiences after treatment according to the postoperative FSFI evaluations. Huebner et al. (24) also suggested psychological counseling as part of the treatment plan for patients with MRKHS. Most patients with congenital absence of the vagina are mentally distressed due to the physical defect (25). MVLESS conceals the surgical incision in or around the umbilicus with little to no scarring. Therefore, MVLESS has excellent postoperative cosmetic results. As a result, the patient’s self-confidence and self-evaluation are significantly improved after surgery.
At present, nationally and internationally, there is no radical cure for patients with congenital absence of the vagina. The ideal treatment is to create an artificial vagina that is anatomically and functionally similar to a normal vagina and to resolve the patient’s sexual challenges with a vaginoplasty. We found that the MVLESS method was better than the TMV method in terms of sexual function, VAS, total FSFI score, and FGSIS score. Although both TMV and MVLESS produced good anatomical and functional results in the treatment of vaginal agenesis, the MVLESS method is less complex than the TMV technique and should be recommended for all MRKHS patients. The perfect combination of vaginoplasty and MVLESS helps patients to realize their goal of normal sexual activity and also gives the patient optimism about having their own children through assisted reproductive technology or surrogacy (3,26).
The generalizability of this article is limited because it was conducted at a single center with a small number of patients; it is uncertain whether the patients studied are representative of all patients.
Conclusions
The MVLESS procedure is a simple, safe, and minimally invasive procedure that provides an anatomical and functional neovagina for MRKHS patients. And it could be used as an alternative for creating a neovagina to achieve satisfactory anatomical and sexual function. However, it is still a difficult problem to solve fertility problems for patients with congenital absence of vagina.
Acknowledgments
Funding: This study was supported by the Promotion Project of Appropriate Technology for Transvaginal Surgery (No. 2020SF-307).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4360/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4360/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4360/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Xi’an People’s Hospital (Xi’an Fourth Hospital) (No. 20220117), and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fontana L, Gentilin B, Fedele L, et al. Genetics of Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome. Clin Genet 2017;91:233-46. [Crossref] [PubMed]
- Brucker SY, Pösch LS, Graf J, et al. Rare genital malformations in women's health research: sociodemographic, regional, and disease-related characteristics of patients with Mayer-Rokitansky-Küster-Hauser syndrome. BMC Womens Health 2020;20:135. [Crossref] [PubMed]
- Committee on Adolescent Health Care. ACOG Committee Opinion No. 728: Müllerian Agenesis: Diagnosis, Management, And Treatment. Obstet Gynecol 2018;131:e35-42. [Crossref] [PubMed]
- Chaikof M, McDermott CD, Brennand E, et al. Patients Seeking "Vaginoplasty" Deserve Assessment and Treatment by Experts in Female Pelvic Medicine and Reconstructive Surgery. Aesthet Surg J 2021;41:NP148-9. [Crossref] [PubMed]
- Navarro V, Acién MI, Acién P. Classical McIndoe Technique Versus the McIndoe Technique with a Neovaginal PACIENA Prosthesis® and No Skin Graft. J Clin Med 2020;9:3648. [Crossref] [PubMed]
- Sanchez-Ferrer ML, Grimbizis G, Nisolle M, et al. Could Training in an Anatomical Model Be Useful to Teach Different Neovagina Surgical Techniques? A Descriptive Study about Knowledge and Experience of Techniques for Neovagina Surgery. J Clin Med 2020;9:3722. [Crossref] [PubMed]
- Pariser JJ, Kim N. Transgender vaginoplasty: techniques and outcomes. Transl Androl Urol 2019;8:241-7. [Crossref] [PubMed]
- Zak PW, Chow I, Zhu X, et al. The Use of a Hartmann's Pouch for Bowel Vaginoplasty: A Case Report. Plast Reconstr Surg Glob Open 2021;9:e3546. [Crossref] [PubMed]
- Vecchietti G. Neovagina nella syndrome di in RokitanskiKuster-Hauser. Attualita Obstet Ginecol 1965;11:131-47.
- Carvalho JA, Nunes PT, Antunes H, et al. Transumbilical laparoendoscopic single-site adrenalectomy: A feasible and safe alternative to standard laparoscopy. Arch Ital Urol Androl 2019;91:1-4. [Crossref] [PubMed]
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191-208. [Crossref] [PubMed]
- Herbenick D, Reece M. Development and validation of the female genital self-image scale. J Sex Med 2010;7:1822-30. [Crossref] [PubMed]
- Adamiak-Godlewska A, Skorupska K, Rechberger T, et al. Urogynecological and Sexual Functions after Vecchietti Reconstructive Surgery. Biomed Res Int 2019;2019:2360185. [Crossref] [PubMed]
- Pontecorvi P, Bernardini L, Capalbo A, et al. Protein-protein interaction network analysis applied to DNA copy number profiling suggests new perspectives on the aetiology of Mayer-Rokitansky-Küster-Hauser syndrome. Sci Rep 2021;11:448. [Crossref] [PubMed]
- Herlin MK, Petersen MB, Brännström M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: a comprehensive update. Orphanet J Rare Dis 2020;15:214. [Crossref] [PubMed]
- Zhang Y, Deng S, Zhang Y. Comparison of sensitivity and specificity of three-dimensional pelvic floor ultrasound and conventional ultrasound in pelvic floor assessment after delivery. Am J Transl Res 2021;13:12026-33. [PubMed]
- Bayer A, Heinze T, Alkatout I, et al. Embryological Development and Topographic Anatomy of Pelvic Compartments-Surgical Relevance for Pelvic Lymphonodectomy. J Clin Med 2021;10:708. [Crossref] [PubMed]
- Acién P, Nohales-Alfonso FJ, Sánchez-Ferrer ML, et al. Clinical pilot study to evaluate the neovaginal PACIENA prosthesis® for vaginoplasty without skin grafts in women with vaginal agenesis. BMC Womens Health 2019;19:144. [Crossref] [PubMed]
- Peters HE, Johnson BN, Ehli EA, et al. Low prevalence of male microchimerism in women with Mayer-Rokitansky-Küster-Hauser syndrome. Hum Reprod 2019;34:1117-25. [Crossref] [PubMed]
- Obeidat RA, Aleshawi AJ, Tashtush NA, et al. Unicornuate uterus with a rudimentary non-communicating cavitary horn in association with VACTERL association: case report. BMC Womens Health 2019;19:71. [Crossref] [PubMed]
- Moegni F, Meutia AP, Kouwagam AD, et al. Secondary pyosalpinx after reconstructive surgery of vaginal agenesis patient with bilateral hematosalpinx: A case report. Int J Surg Case Rep 2021;85:106166. [Crossref] [PubMed]
- Fedele L, Busacca M, Candiani M, et al. Laparoscopic creation of a neovagina in Mayer-Rokitansky-Küster-Hauser syndrome by modification of Vecchietti's operation. Am J Obstet Gynecol 1994;171:268-9. [Crossref] [PubMed]
- Kim DY, Nam G, Lee SR, et al. Congenital Obstructive Müllerian Anomaly: The Pitfalls of a Magnetic Resonance Imaging-Based Diagnosis and the Importance of Intraoperative Biopsy. J Clin Med 2021;10:2414. [Crossref] [PubMed]
- Huebner M, DeLancey JOL, Reisenauer C, et al. Magnetic resonance imaging of vaginal support structure before and after Vecchietti procedure in women with Mayer-Rokitansky-Küster-Hauser syndrome. Acta Obstet Gynecol Scand 2018;97:830-7. [Crossref] [PubMed]
- Sandman L, Hansson E. An ethics analysis of the rationale for publicly funded plastic surgery. BMC Med Ethics 2020;21:94. [Crossref] [PubMed]
- Georgopapadakos N, Manoli A, Passia G, et al. Uterus Transplantation as a Therapy Method in Mayer-Rokitansky-Küster-Hauser Syndrome. Cureus 2019;11:e6333. [Crossref] [PubMed]




