
Lipidomics reveals significant alterations associated with exclusive enteral nutrition treatment in adult patients with active Crohn’s disease
Introduction
Crohn’s disease (CD) is an inflammatory bowel disease (IBD), which represents a group of progressive disorders with recurrent chronic intestinal inflammation. Diarrhea, abdominal pain, rectal bleeding, fever, weight loss, exhaustion, growth retardation, anemia, recurring fistulas, or extraintestinal manifestations are typical CD symptoms (1). A transmural, discontinuous inflammatory process, along with focal crypt abnormalities and non-caseous epithelioid granulomas, are revealed by CD histopathology (2). Over the past three decades, the prevalence of CD has increased by almost 40 times worldwide. The incidence is higher in Europe and North America (3.77 to 6.38 cases per 100,000 person-years) and is rising worldwide, particularly in areas with previously low morbidity (Africa, Asia, and South America) (3,4). While CD has become a global disease, its etiology and pathogenesis remain unknown. Genetic susceptibility, dysregulated immune systems, and exposome-triggering factors such as microbial and dietary factors might contribute to CD development (5-7). In recent years, researchers have focused more on the role of dietary and nutritional factors in CD pathogenesis and intervention.
Exclusive enteral nutrition (EEN) is a nutritional treatment with minimal adverse effects. Studies have compared the effect of EEN to that of steroids, which are commonly used as first-line induction therapy for active CD but have several adverse side effects, including Cushing syndrome, acne, infection, and growth delay. Steroids are usually administered orally with a dosage of prednisolone at 40–60 mg/day and, subsequently, tapered by 5 mg/week to a daily dose of 20 mg, followed by a 2.5–5.0 mg weekly tapering schedule with no usage for more than 3 months (1). A meta-analysis including five randomized clinical trials that compared the effect of EEN with corticosteroids suggested that EEN was as efficacious as corticosteroids (8). Studies performed at our center have demonstrated that EEN is equally effective as steroids and biologics for inducing early clinical remission in adult patients with CD, particularly in complicated cases, such as those with intestinal fistulas, abdominal abscesses, inflammatory intestinal strictures, or treatment resistance (9,10). Specifically, EEN promotes mucosal healing (MH) and fistula closure and reduces abdominal abscess size. In the recent CD management guidelines, EEN is recommended as a first-line intervention for adolescent patients and an alternative to steroids and biological drugs for inducing disease remission in adults (11,12). Based on these findings, dietary and nutritional factors have been increasingly recognized as determining factors in CD onset. As such, EEN-mimicking dietary therapies, such as CD-treat or the CD-exclusion diet, have been used as CD interventions. However, these diet interventions are still not comparable to EEN and cannot replace EEN (13,14).
While EEN effectively manages CD, its specific molecular mechanism remains undefined. Genetic predisposition and gene-environment interactions, as well as epigenetic modifications, such as DNA-methylation and non-coding RNAs, might be involved in CD pathogenesis (15). Since diet and nutritional components can both influence the intestinal microflora and regulate gene expression directly or indirectly via epigenetic mechanisms (16,17), analysis of nutritional component alterations after EEN intervention could help clarify its mechanism. Previous studies have reported that EEN potentially inhibits inflammation through influencing the intestinal bacterial milieu and body metabolites (18). Diederen et al collected stool samples from a pediatric population consisting of 43 patients with CD and 15 healthy controls (HCs). They reported that EEN decreased the diversity of microbiota, reduced amino acids, and decreased the levels of trimethylamine and cadaverine towards the control levels. Patients with CD had a reduced microbial metabolism of bile acids that was partially normalized during EEN. They further performed in vivo and in vitro experiments which confirmed that trimethylamine, cadaverine, bile salts, and amino acids could play a role in the mechanism by which EEN induces remission (19). Lipids are essential nutritional substances for life activities, as they are crucial for signal transduction, cell membrane composition, and energy storage (20). Significant changes in lipid metabolism are associated with CD; lipid metabolism and signaling are thought to play important roles in inflammation, with significant implications for IBD (21-24). Previous studies mainly focused on the clinical effect of EEN or its effect on the microbial changes in CD. Studies that investigated lipidomics in CD mainly focused on the role of the lipids in the etiology and pathogenesis of CD or its diagnostic value for the differential diagnosis of CD. The analysis of the changes in the lipid profile after EEN treatment in CD is lacking and has not been systematically investigated before.
Therefore, we conducted a comprehensive lipidomic analysis of 869 plasma lipid species using ultra-high performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) to determine whether plasma lipid profile changes occur during EEN intervention and their relationship with EEN in CD. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4225/rc).
Methods
Participants
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (approval No. 2022ZSLYEC-019). All participants provided informed consent before they were enrolled in the study.
A total of 11 adult patients with active CD and 17 age- and sex-matched HCs were recruited from March 2020 to December 2020. The inclusion criteria were age ≥18 years; a CD diagnosis using international criteria (1); active luminal disease confirmed with symptoms on endoscopy and computed tomography enterography (CTE)/magnetic resonance enteroclysis (MRE); and 12-week enteral nutrition formula treatment without any other food intake. The exclusion criteria were a history of hyperlipidemia or antibiotic treatment within 2 weeks; concurrent antibiotic or anti-inflammatory agent treatment (such as steroids, thiopurines, methotrexate, and thalidomide); and patients who had ever been diagnosed with cancer or immune system diseases. Patients received enteral nutrition formulas such as Elental (Ajinomoto Pharma, Tokyo, Japan), Enteral Nutritional Suspension (SP) (NUTRICIA, New Zealand), or Enteral Nutritional Emulsion (TP) (Fresenius Kabi Deutschland GmbH, Germany), all of which are commonly used enteral nutrition formulas for the treatment of CD in China. A sufficient amount of individual daily energy supplementation was calculated for each patient according to previous studies (25,26), and the patients were followed up every 4 weeks at our center.
Data on the participants’ demographics, including family history, disease location and behavior, intestinal surgery, perianal disease, intestinal fistula/abscess, and medication history, are presented in Table 1. The clinical classifications of CD were assessed using the Montreal Classification (27). Endoscopy was performed at baseline and 12 weeks after the EEN treatment. The simple endoscopic score for CD (SES-CD) was used to evaluate MH (indicated by a score <2) (28). Disease activity was assessed at baseline and week 12 using the Crohn’s Disease Activity Index (CDAI). A CDAI score <150 was considered clinical remission (29). Serum inflammatory indices, such as high-sensitivity C-reactive protein (hs-CRP), platelets (PLTs), and the erythrocyte sedimentation rate (ESR), as well as nutrition-associated markers, such as body mass index (BMI), albumin (ALB), hemoglobin (Hb), and the European Nutritional Risk Screening 2002 (NRS 2002) score, were examined at baseline and week 12.
Table 1
| Characteristics | Crohn’s disease (n=11) |
|---|---|
| Gender | |
| Male | 6 |
| Female | 5 |
| Age (years), mean ± SD | 25.36±4.84 |
| Family history, n (%) | |
| No | 11 (100.0) |
| Behavior, n (%) | |
| B1 | 5 (45.5) |
| B2 | 1 (9.1) |
| B3 | 5 (45.5) |
| Location, n (%) | |
| L1 | 0 |
| L2 | 0 |
| L3 | 9 (81.8) |
| L4 | 2 (18.2) |
| Intestinal surgery, n (%) | |
| Yes | 1 (9.1) |
| No | 10 (90.9) |
| Perianal disease, n (%) | |
| Yes | 9 (81.8) |
| Intestinal fistula/abscess, n (%) | |
| Yes | 5 (45.5) |
| No | 6 (54.5) |
| Medication history, n (%) | |
| Steroids | 0 |
| Mesalazine | 3 (27.3) |
| Thiopurine | 4 (36.4) |
| Cyclosporine | 0 |
| Anti-TNF-α agent | 4 (36.4) |
| Methotrexate | 0 |
| Thalidomide | 0 |
B1, non-stenotic and non-penetrating behavior; B2, stenotic behavior; B3, penetrating behavior; L1, terminal ileum; L2, colon; L3, ileum colon; L4, upper gastrointestinal tract; TNF, tumor necrosis factor; SD, standard deviation.
Lipidomic analysis
Sample preparation and lipid extraction
After overnight fasting, blood samples were obtained from HCs and patients with CD before EEN and 12 weeks after EEN. The plasma fraction was obtained by centrifuging the samples and was kept at −80 ℃ for further use. Lipids were extracted using the methyl-tert-butyl ether (MTBE) method.
Lipid analysis method using liquid chromatography (LC)-MS/MS
The charged surface hybrid (CSH) C18 column (1.7 µm, 2.1 mm, 100 mm; Waters, Milford, Massachusetts, USA) was chosen for the LC separation utilizing reverse phase chromatography. Then, 3 µL of the sample was injected after the lipid extracts were redissolved in 200 L of 90% isopropanol/acetonitrile and centrifuged at 14,000 g for 15 min. Acetonitrile-water (6:4 v/v), which also contained 0.1 mM ammonium formate and 0.1% formic acid, served as solvent A, while acetonitrile-isopropanol (1:9 v/v) with 0.1% formic acid and 0.1 mM ammonium formate served as solvent B. The initial mobile phase was 30% solvent B at a flow rate of 300 µL/min. After equilibrating at 5% solvent B for 10 min, it was linearly raised to 100% solvent B in 23 min.
Q-Exactive Plus (Thermo Fisher Scientific, USA) was used to acquire mass spectra in both positive and negative modes. The electron spray ionization (ESI) parameters were optimized and preset for all measurements as follows: source temperature, 300 ℃; capillary temperature, 350 ℃; ion spray voltage, 3,000 V; S-Lens radio frequency level, 50%; scanning range, 200–1,800 m/z.
LipidSearch-based identification
Based on the MS/MS calculations, LipidSearch software (Thermo Fisher Scientific, USA) was utilized to identify the lipid species. The mass tolerance for both the precursor and the fragment was set to 5 ppm.
Statistical analysis
The quantitative data are expressed as the mean ± standard deviation. Paired t-tests and Chi-squared/Fisher’s exact tests were used to compare the continuous and categorical variables before and after EEN, respectively. The correlation between clinical and lipid parameters was analyzed using Pearson and Spearman correlations. P<0.05 was considered statistically significant. All the measurements were analyzed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
The LipidSearch-extracted data were analyzed with univariate, multivariate, hierarchical cluster, and correlation analyses. The univariate statistical analysis included a student’s t-test, nonparametric test, and fold change (FC) analysis. FC >1.5 or <0.67 and P<0.05 indicated differential lipid molecules in the univariate analysis. After data preprocessing with Pareto-scaling, a multidimensional statistical analysis was performed using SIMCA-P 14.1 (Umetrics, Umea, Sweden). An orthogonal partial least squares discriminant analysis (OPLS-DA) was used to determine the global metabolic changes between groups. The variable importance in the projection (VIP) was calculated for the OPLS-DA model. VIP >1 and P<0.05 indicated differential lipid molecules.
The significantly altered functional pathways were visualized with an ingenuity pathway analysis (IPA) using MetaboAnalyst 4.0 and MetScape, which are freely available online tools.
Results
Participants’ demographics
From March 2020 to December 2020, 13 adult patients with active CD accepted the EEN intervention, but two patients withdrew due to intolerance of the treatment. Finally, 11 patients with CD and 17 age- and sex-matched HCs (5 women, 12 men; mean age, 27.35±4.35 years) were recruited. Their detailed demographic data are summarized in Table 1.
Changes in clinical index, clinical remission, and MH after 12 weeks of EEN
Among the 11 patients with active CD, 9 (81.8%) achieved remission and demonstrated a significant decrease in the CDAI from 291.09±78.57 before the treatment to 118.09±79.11 after the treatment (P<0.001; Table S1). One patient with a clinical response did not achieve remission; another with no response to EEN treatment received another therapy. Ten patients had an SES-CD score >2 before the treatment, and 4 (40.0%) achieved MH after the treatment (SES-CD score 6.45±2.81 vs. 3.18±2.22, P=0.013; Table S1). After 12 weeks of EEN, hs-CRP (pre-treatment vs. post-treatment: 64.94±38.38 vs. 6.54±16.26 mg/L; P=0.001), ESR (62.64±28.43 vs. 17.55±4.33 mm/h; P<0.001), and PLT [(463.00±136.88)×109/L vs. (298.36±107.75)×109/L; P=0.001] decreased significantly. The average BMI (17.05±2.02 vs. 18.64±2.36 kg/m2; P=0.005) significantly improved, and the NRS 2002 score significantly decreased (3.91±1.14 vs. 2.55±1.29; P<0.001). ALB and Hb also improved (Table S1). These significant changes suggested a less active disease and an improved nutrition status in patients after the EEN intervention.
Plasma lipid profiles at the lipid class level
Lipid class alterations in HCs and patients before and after EEN
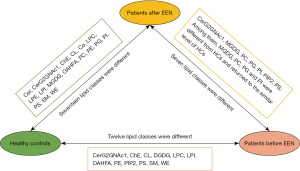
A total of 869 lipid species were measured using UHPLC-MS/MS and grouped into 28 classes (Figure S1). Before treatment, 17 lipid classes differed between the CD and HC groups. After the treatment, 12 classes showed differences between the two groups (P<0.05, Figure 1). The plasma levels of seven lipid classes in patients with CD changed significantly after EEN treatment, with increases in simple glucosylceramide series (CerG2GNAc1), monogalactosyldiacylglycerol (MGDG), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylcholine (PC) and decreases in phosphatidylglycerol (PG) and phosphatidylinositol diphosphate (PIP2). Notably, MGDG, PI, and PC levels were lower, and the PG level was higher in CD patients before EEN treatment than HCs (P<0.05, Figure 2). These levels normalized to HC levels after EEN treatment (P>0.05).
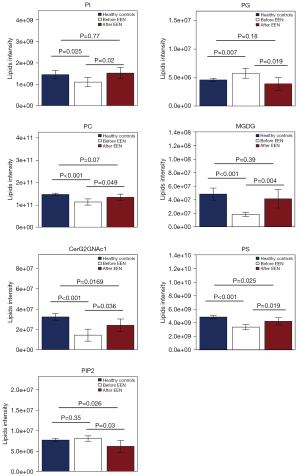
Associations between the lipid class levels and the clinical indices
Correlations between the lipid classes and the disease activity/inflammatory markers (CDAI, PLT, hs-CRP, ESR, and SES-CD) in CD patients were analyzed. While PI, MGDG, CerG2GNAc1, PC, and PS levels negatively correlated with the CDAI, PG levels positively correlated with the CDAI. PG and PS levels negatively correlated with PLT, while CerG2GNAc1 levels positively correlated with PLT. MGDG and CerG2GNAc1 levels negatively correlated with hs-CRP, while PG levels positively correlated with hs-CRP. PI, MGDG, CerG2GNAc1, PC, and PS levels were negatively associated with ESR. CerG2GNAc1 and PS levels negatively correlated with SES-CD (Table 2).
Table 2
| Disease activity and inflammatory markers | Lipid classes | ||||||
|---|---|---|---|---|---|---|---|
| PI | MGDG | CerG2GNAc1 | PC | PG | PS | PIP2 | |
| CDAI | −0.575** | −0.521* | −0.425* | −0.472* | 0.476* | −0.574** | 0.442* |
| P value | 0.005 | 0.013 | 0.048 | 0.026 | 0.025 | 0.005 | 0.039 |
| PLT | −0.319 | −0.315 | −0.478* | −0.420 | 0.477* | −0.478* | 0.474* |
| P value | 0.148 | 0.153 | 0.025 | 0.051 | 0.025 | 0.025 | 0.026 |
| hs-CRP | −0.405 | −0.481* | −0.468* | −0.215 | 0.591** | −0.318 | 0.232 |
| P value | 0.062 | 0.023 | 0.028 | 0.337 | 0.004 | 0.149 | 0.300 |
| ESR | −0.513* | −0.465* | −0.584** | −0.451* | 0.342 | −0.488* | 0.352 |
| P value | 0.015 | 0.029 | 0.004 | 0.035 | 0.119 | 0.021 | 0.108 |
| SES-CD | −0.287 | −0.331 | −0.442* | −0.288 | 0.208 | −0.480* | 0.087 |
| P value | 0.195 | 0.132 | 0.039 | 0.194 | 0.353 | 0.024 | 0.701 |
*, P<0.05; **, P<0.01. CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CerG2GNAc1, simple glucosylceramide series; ESR, erythrocyte sedimentation rate; hs-CRP, high-sensitivity C-reactive protein; MGDG, monogalactosyldiacylglycerol; PC, phosphatidylcholine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PIP2, phosphatidylinositol diphosphate; PLT, platelet; PS, phosphatidylserine; SES-CD, simple endoscopic score for Crohn’s disease.
Plasma lipid profiles at the lipid species level
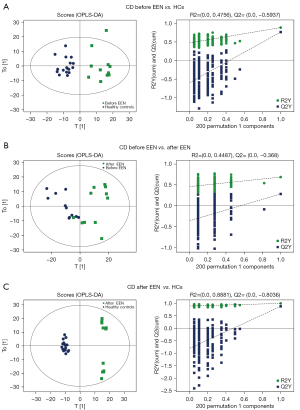
Three OPLS-DA models were used for the three pair-wise analyses of the lipid groups among CD patients and HCs pre- and post-treatment. All models displayed a clear separation among the different groups and passed the permutation test (Figure 3). Samples from each group were significantly separated, indicating that the lipid metabolism changed significantly due to the disease status itself and the EEN treatment. These models showed good reliability. The Q2 of the OPLS-DA for CD patients pre- vs. post-treatment was 0.272, which could be partially explained by obvious differences in the disease status of each patient.
Species-level alterations in plasma lipids
The different lipid species (VIP >1.0, P<0.05) were selected based on the OPLS-DA model. Before EEN treatment, 80 lipid species significantly differed between CD patients and HCs (table available at https://cdn.amegroups.cn/static/public/atm-22-4225-01.pdf). After EEN treatment, 103 lipid species were significantly different between CD patients and HCs (table available at https://cdn.amegroups.cn/static/public/atm-22-4225-02.pdf). Significant changes in 38 lipid species, primarily belonging to the PC, triglyceride (TG), LysoPC (LPC), and PI classes, were observed between CD patients pre- vs. post-treatment (Table 3), indicating that EEN significantly altered the lipid metabolism.
Table 3
| Lipid ion | Class | Ion formula | Fold change | P value | VIP |
|---|---|---|---|---|---|
| ChE(20:4)+NH4 | ChE | C47 H80 O2 N1 | 0.62 | 0.016 | 1.64 |
| PC(32:1)+H | PC | C40 H79 O8 N1 P1 | 4.41 | 0.006 | 4.64 |
| PC(33:1)+H | PC | C41 H81 O8 N1 P1 | 3.94 | 0.019 | 1.33 |
| PC(34:3)+H | PC | C42 H79 O8 N1 P1 | 4.70 | 0.034 | 1.32 |
| PC(34:1)+H | PC | C42 H83 O8 N1 P1 | 1.49 | 0.019 | 5.35 |
| PC(36:4)+H | PC | C44 H81 O8 N1 P1 | 2.83 | 0.006 | 1.57 |
| PC(36:3)+H | PC | C44 H83 O8 N1 P1 | 6.67 | 0.007 | 5.32 |
| PC(36:1)+H | PC | C44 H87 O8 N1 P1 | 1.96 | 0.021 | 3.73 |
| LPC(16:0)+H | LPC | C24 H51 O7 N1 P1 | 1.50 | 0.024 | 1.06 |
| PC(38:7)+H | PC | C46 H79 O8 N1 P1 | 0.83 | 0.014 | 1.15 |
| PC(16:0/22:6)+H | PC | C46 H81 O8 N1 P1 | 4.31 | 0.007 | 1.19 |
| PC(38:3)+H | PC | C46 H87 O8 N1 P1 | 6.17 | 0.013 | 3.23 |
| TG(16:0/14:0/18:2)+NH4 | TG | C51 H98 O6 N1 | 4.89 | 0.021 | 3.30 |
| TG(16:0/16:0/16:1)+NH4 | TG | C51 H100 O6 N1 | 5.16 | 0.031 | 3.78 |
| TG(16:0/16:0/16:0)+NH4 | TG | C51 H102 O6 N1 | 3.46 | 0.023 | 1.77 |
| PC(40:6)+H | PC | C48 H85 O8 N1 P1 | 5.54 | 0.010 | 1.27 |
| TG(16:0/16:1/18:1)+NH4 | TG | C53 H102 O6 N1 | 2.47 | 0.034 | 5.82 |
| TG(16:0/16:0/18:1)+NH4 | TG | C53 H104 O6 N1 | 2.34 | 0.021 | 4.30 |
| TG(18:0/16:0/16:0)+NH4 | TG | C53 H106 O6 N1 | 3.56 | 0.031 | 1.63 |
| TG(16:0/17:1/18:1)+NH4 | TG | C54 H104 O6 N1 | 2.40 | 0.045 | 1.38 |
| LPC(16:1)+HCOO | LPC | C25 H49 O9 N1 P1 | 3.33 | 0.013 | 1.54 |
| LPC(16:0)+HCOO | LPC | C25 H51 O9 N1 P1 | 1.42 | 0.030 | 1.78 |
| PE(16:1/18:1)-H | PE | C39 H73 O8 N1 P1 | 2.01 | 0.019 | 1.72 |
| PE(16:0/20:4)-H | PE | C41 H73 O8 N1 P1 | 2.27 | 0.017 | 1.89 |
| PE(18:1/18:2)-H | PE | C41 H75 O8 N1 P1 | 2.10 | 0.036 | 1.22 |
| PC(14:0/18:2)+HCOO | PC | C41 H77 O10 N1 P1 | 2.39 | 0.022 | 1.00 |
| PC(16:0/16:1)+HCOO | PC | C41 H79 O10 N1 P1 | 4.75 | 0.005 | 4.86 |
| PC(17:1/16:0)+HCOO | PC | C42 H81 O10 N1 P1 | 3.78 | 0.021 | 1.45 |
| PC(16:0/18:1)+HCOO | PC | C43 H83 O10 N1 P1 | 1.60 | 0.017 | 7.12 |
| PI(16:0/16:1)-H | PI | C41 H76 O13 N0 P1 | 9.34 | 0.005 | 1.89 |
| PC(18:2/18:2)+HCOO | PC | C45 H81 O10 N1 P1 | 2.80 | 0.005 | 1.62 |
| PS(39:2)-H | PS | C45 H83 O10 N1 P1 | 6.07 | 0.008 | 6.08 |
| PC(18:0/18:1)+HCOO | PC | C45 H87 O10 N1 P1 | 1.92 | 0.022 | 4.22 |
| PI(16:0/18:1)-H | PI | C43 H80 O13 N0 P1 | 4.07 | 0.005 | 2.70 |
| PC(18:0/20:2)+HCOO | PC | C47 H89 O10 N1 P1 | 1.52 | 0.016 | 1.08 |
| PI(16:0/20:3)-H | PI | C45 H80 O13 N0 P1 | 10.84 | 0.008 | 1.32 |
| MGDG(43:10)-H | MGDG | C52 H79 O10 | 2.23 | 0.004 | 1.05 |
| PI(18:0/20:3)-H | PI | C47 H84 O13 N0 P1 | 10.03 | 0.033 | 2.62 |
ChE, cholesterol ester; LPC, lysophosphatidylcholine; MGDG, monogalactosyldiacylglycerol; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; TG, triglyceride; VIP, variable importance in the projection; CD, Crohn’s disease; EEN, exclusive enteral nutrition.
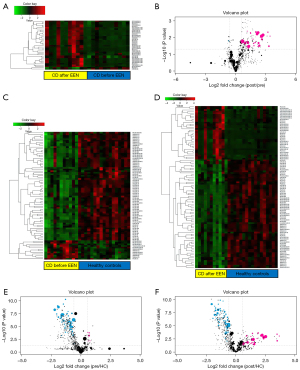
To show the relationship between the different samples and the differences in the lipid expression patterns in the samples, we used the expression of qualitatively significant differential lipids, with VIP >1 and P<0.05, to conduct hierarchical clustering for each sample group. As shown in the heatmap (Figure 4A), the plasma lipid species before and after the EEN intervention can be divided into two large groups, which may play an important role in the EEN mechanism and help distinguish its efficacy. The volcano plot analysis, based on VIP, P, and FC values, showed different lipid species in CD patients at pre- vs. post-intervention (Figure 4B). The hierarchical clustering analysis and volcano plots for CD patients before and after the EEN intervention compared to HCs also suggested lipid species among the different groups were altered (Figure 4C-4F). Correlation analysis was performed to further understand the interrelationships of the significantly altered lipid molecules, and the results suggested significant interrelationships among them (Figure 5).
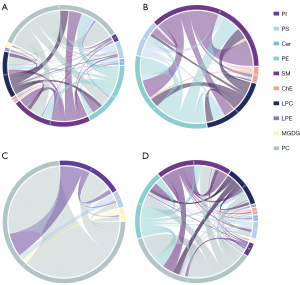
Correlations between the lipid species and clinical indices
We analyzed the correlations between the lipid species and the disease activity/inflammatory markers in CD patients. As with the lipid classes, many lipid species negatively or positively correlated with the CDAI, hs-CRP, ESR, and SES-CD (table available at https://cdn.amegroups.cn/static/public/atm-22-4225-03.pdf), further confirming that they may be involved in the EEN mechanism.
Altered metabolic pathways
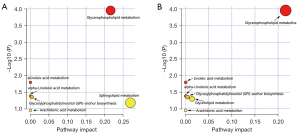
IPA was performed to understand the function of the altered lipid species. Compared to HCs, the top five dysregulated lipid pathways in pre-intervention CD patients correlated with the metabolism of glycerophospholipids, sphingolipids, linolenic acid, and arachidonic acid (AA), as well as the glycosylphosphatidylinositol (GPI) anchor biosynthesis (Figure 6A). The functional pathways potentially related to EEN treatment, including the metabolism of glycerophospholipids, linolenic acid, glycerolipids, and AA, as well as the biosynthesis of the GPI-anchor, were also analyzed (Figure 6B). Furthermore, the network of the significantly altered metabolomic data related to the differential lipid species was also summarized by MetScape analysis, which suggested that the metabolism of AA, glycerophospholipids, linoleate, and PI phosphate was significantly altered in patients receiving EEN treatment (Figure S2).
Discussion
This study focused extensively on plasma lipid profiling of patients with CD who received EEN treatment. Significant alterations in the plasma lipids of CD patients were observed after EEN intervention. Additionally, the levels of lipid classes and species in these patients significantly correlated with the CDAI, SES-CD, and various inflammatory biomarkers (hs-CRP, PLT, and ESR). A pathway analysis further revealed that the metabolism of AA, glycerophospholipids, linoleate, and PI phosphate was altered in CD patients, suggesting an association between these pathways and EEN.
Previous studies have shown significant alterations in the lipid profiles of patients with CD. Fan et al. (30) reported changes in 33 lipid species (mostly ether lipids, such as alkylphospholipids and plasmalogens) in CD patients compared to controls. Scoville et al. revealed that 162 serum lipid species were significantly altered in CD patients compared to controls. The levels of the long chain, polyunsaturated, branched chain, and monohydroxy fatty acids were consistently reduced in these patients. Additionally, the levels of conjugated and unconjugated primary and secondary bile acids were elevated in CD patients compared to controls (31). In a study of plasma lipid profiles of Italian IBD patients, Murgia et al. showed that levels of LPC 18:2, PC 18:0/18:1, PC 18:1/18:1, PC 18:2/18:2, and PC 18:3/20:4 were lower in CD patients than controls, while levels of PC 16:0/22:6, PC 18:0/22:6, PC 18:3/20:3, sphingomyelin (SM) 16:1/20:1, TG 18:1/18:2/20:4, and TG 16:0/18:1/18:2 were higher (32). A recent study also revealed that plasma levels of lysophosphatidylserine, PS, and sphingosine-1-phosphate were significantly higher in CD patients than in controls, while those of lysophosphatidylinositol (LPI) and PC were significantly lower (22). We observed that 17 classes [ceramide (Cer), CerG2GNAc1, cholesterol ester (ChE), cardiolipins (CL), coenzyme (Co), LPC, lysophosphatidylethanolamine (LPE), LPI, MGDG, (O-acyl)-1-hydroxy fatty acid (OAHFA), PC, phosphatidylethanolamine (PE), PG, PI, PS, SM, and wax esters (WE)] and 80 lipid species were significantly different between CD patients before EEN intervention and HCs. Changes in some of these lipid classes have also been reported in previous studies (22,30-32). However, some lipids in our study were not previously investigated, and the results of different studies have been inconsistent. This may be partly due to differences in the sample collection methods and study populations (such as age and sex distributions, disease activity, dietary profiles, medications, etc.). In our study, the patients with CD were relatively young and exhibited active disease without concomitant drug use. Furthermore, the methods of lipids extraction and separation that differ among various studies may influence the results. Currently, a variety of technical methods are applied in lipid analysis, including gas chromatography-mass spectrometry (GC-MS), LC-mass spectrometry (LC-MS), and nuclear magnetic resonance. The nuclear magnetic resonance method, with low sensitivity, can only be used in tissues with high content of detectable lipids. Analysis using GC-MS is limited to the samples that can be vaporized and ionized at approximately 300 ℃ or lower. In comparison to GC-MS, LC-MS is suitable for analyzing polar and non-polar compounds. UHPLC is composed of a fixed loaded column with particle size less than 2 µm, high pressure solvent output adapted to its speed and performance, fast automatic prototype, detector, data acquisition control system, and low dead volume chromatography system that can withstand higher pressure. The UHPLC-MS/MS has higher sensitivity, separation, and analysis speed than does the conventional LC-MS. Thus, UHPLC-MS/MS represents a powerful tool for lipidomic studies and has been widely used in this field (33,34). Accordingly, we choose the UHPLC-MS/MS method to accurately analyze the plasma lipids.
Potential plasma lipid alterations induced by EEN intervention in patients with CD have not been investigated. Our results suggest that seven lipid classes (CerG2GNAc1, MGDG, PG, PC, PI, PIP2, and PS) and 38 lipid species were significantly different at pre- vs. post-intervention in CD patients. Among the seven lipid classes, CerG2GNAc1, MGDG, PI, PS, and PC levels increased significantly after EEN intervention in CD patients, while PG and PIP2 levels decreased significantly. Additionally, some lipid classes and species significantly correlated with hs-CRP, CDAI, SES-CD, PLT, and ESR (Table 2 and table available at https://cdn.amegroups.cn/static/public/atm-22-4225-03.pdf), suggesting that lipids may be involved in the mechanism of EEN. For example, the negative correlations of PS with CDAI, PLT, ESR, and SES-CD suggested that PS may inhibit inflammation in CD. A recent investigation of the role of PS in experimental colitis revealed that the anti-inflammatory effect of Annexin A5 depended on PS endocytosis, suggesting externalized PS on colonic capillaries may be utilized as a novel pharmacological target for IBD (35). Another study also reported that an increased endothelial PS level might promote a hypercoagulable state, resulting in a thrombosis risk for IBD patients (36). A higher PI level significantly correlated with a lower CDAI and ESR (Table 2). PI was transformed into its derivatives in the body. Moreover, inositol trisphosphate (IP3) enema, a PI derivative, promoted recovery from intestinal inflammation and increased the lifespan of mice with experimental colitis by stimulating the repair of intestinal epithelial cells by regulating intestinal histone deacetylase 3 activity (37). In summary, our study showed a correlation between identified lipid classes and species and disease activity markers, which should be confirmed in future studies with larger sample sizes and validated via mechanistic investigations.
The IPA revealed that the metabolism of glycerophospholipids, AA, linoleate, and PI phosphate was significantly altered in CD patients receiving EEN. These pathways may be involved in the EEN mechanism or CD pathogenesis. Notably, lysophosphatidylcholines (LPCs) derived from glycerophospholipids were shown to be differentially expressed in the plasma samples of rats with experimental colitis. LPCs were associated with intestinal barrier function damage by increasing ileal permeability (38). Previous studies have systematically reviewed the role of AA metabolites, including prostaglandins, thromboxanes, lipoxygenases, and leukotrienes, in IBD (39,40). These metabolites showed differential expressions in the diseased intestinal mucosa in IBD patients and immunomodulatory roles in animal studies. Although increasing evidence has shown that these lipid pathways may play pivotal roles in the inflammatory process in animal and in vitro experiments, their influence on EEN intervention in humans remains unclear, given the complexity of the interactions among the mixture of lipids and other compounds in the human body. Furthermore, the mechanistic impact of EEN on inducing changes in lipid metabolism is still unclear. Previous studies reported that nutrients present in the enteral formulas could regulate the lipid metabolism. For instance, carbohydrates, such as dextrin, are abundant in the enteral nutrition formulas and can be converted into fats in the body to regulate the lipid metabolism. Omega 3 fatty acids are also present in the enteral nutrition formulas. A randomized double-blinded placebo-controlled trial revealed that the high-dose intake of omega 3 fatty acids combined with statins has a favorable role in altering the levels of serum TGs and non-high density lipoprotein cholesterol in patients with hyperlipidemia (41). Xiong et al. reported that a high-iron diet could regulate lipid metabolism by significantly increasing the expression of the fat hydrolysis enzyme genes in both the liver and adipose tissues in mice (42). Han et al. suggested that microbiota depletion in high-fat diet-fed mice changed the expression of the hepatic genes involved in cholesterol and fatty acid metabolism (43). Accordingly, the nutrients in EEN and the changes in the EEN-induced gut microbiota may regulate the lipid metabolism through affecting gene expression, endogenous transformation, or the other mechanisms. Thus, the exact mechanism of EEN on altering lipid metabolism needs further studies. Further studies are required to explore these topics.
This study had some limitations. First, the population of CD patients treated with EEN was small, partly due to the low incidence of CD. This small sample size prevented us from performing additional analyses, such as biomarker efficacy screening. Therefore, more comprehensive studies with larger sample sizes are required. Second, only correlational analyses of lipid alterations were performed, and their clinical significance needs further confirmation. Third, our results are based on bioinformatics analysis. Whether these lipid alterations were involved in the mechanism of EEN or were simply EEN outcomes also remains undetermined. Further in vitro and in vivo experimental studies are required to confirm our results and the relationship between EEN and lipids.
Conclusions
EEN induces alterations in multiple lipid classes and species, leading to improvements in clinical response. EEN primarily altered the AA, glycerophospholipids, linoleate, and PI phosphate lipid pathways. Further studies are needed to confirm our results and the precise roles of these lipids in EEN diet therapy.
Acknowledgments
Funding: This work was supported by the National Nature Science Fund of China (Nos. 81870382 and 81700495) and the Youth Project of the Natural Science Foundation of China (No. 82100547).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4225/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4225/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4225/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (approval No. 2022ZSLYEC-019), and all participants provided informed consent before they were enrolled in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol 2018;113:481-517. [Crossref] [PubMed]
- Adamina M, Feakins R, Iacucci M, et al. ECCO Topical Review Optimising Reporting in Surgery, Endoscopy, and Histopathology. J Crohns Colitis 2021;15:1089-105. [Crossref] [PubMed]
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769-78. [Crossref] [PubMed]
- Park SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res 2022;20:159-64. [Crossref] [PubMed]
- Sartor RB. Microbial and dietary factors in the pathogenesis of chronic, immune-mediated intestinal inflammation. Adv Exp Med Biol 2006;579:35-54. [Crossref] [PubMed]
- Boyapati R, Satsangi J, Ho GT. Pathogenesis of Crohn's disease. F1000Prime Rep 2015;7:44. [Crossref] [PubMed]
- Petagna L, Antonelli A, Ganini C, et al. Pathophysiology of Crohn's disease inflammation and recurrence. Biol Direct 2020;15:23. [Crossref] [PubMed]
- Heuschkel RB, Menache CC, Megerian JT, et al. Enteral nutrition and corticosteroids in the treatment of acute Crohn's disease in children. J Pediatr Gastroenterol Nutr 2000;31:8-15. [Crossref] [PubMed]
- Yang Q, Tang J, Ding N, et al. Twelve-week peptide-based formula therapy may be effective in inducing remission of active Crohn disease among women who are pregnant or preparing for pregnancy. Nutr Clin Pract 2022;37:366-76. [Crossref] [PubMed]
- Yang Q, Gao X, Chen H, et al. Efficacy of exclusive enteral nutrition in complicated Crohn's disease. Scand J Gastroenterol 2017;52:995-1001. [Crossref] [PubMed]
- Lochs H, Dejong C, Hammarqvist F, et al. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin Nutr 2006;25:260-74. [Crossref] [PubMed]
- Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179-207. [Crossref] [PubMed]
- Svolos V, Hansen R, Nichols B, et al. Treatment of Active Crohn's Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019;156:1354-1367.e6. [Crossref] [PubMed]
- Levine A, Wine E, Assa A, et al. Crohn's Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019;157:440-450.e8. [Crossref] [PubMed]
- Annese V. Genetics and epigenetics of IBD. Pharmacol Res 2020;159:104892. [Crossref] [PubMed]
- Singh V, Vijay-Kumar M. Beneficial and detrimental effects of processed dietary fibers on intestinal and liver health: health benefits of refined dietary fibers need to be redefined! Gastroenterol Rep (Oxf) 2020;8:85-9. [Crossref] [PubMed]
- Gruber L, Lichti P, Rath E, et al. Nutrigenomics and nutrigenetics in inflammatory bowel diseases. J Clin Gastroenterol 2012;46:735-47. [Crossref] [PubMed]
- Hansen T, Duerksen DR. Enteral Nutrition in the Management of Pediatric and Adult Crohn's Disease. Nutrients 2018;10:537. [Crossref] [PubMed]
- Diederen K, Li JV, Donachie GE, et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn's disease. Sci Rep 2020;10:18879. [Crossref] [PubMed]
- Yoon H, Shaw JL, Haigis MC, et al. Lipid metabolism in sickness and in health: Emerging regulators of lipotoxicity. Mol Cell 2021;81:3708-30. [Crossref] [PubMed]
- Guan S, Jia B, Chao K, et al. UPLC-QTOF-MS-Based Plasma Lipidomic Profiling Reveals Biomarkers for Inflammatory Bowel Disease Diagnosis. J Proteome Res 2020;19:600-9. [Crossref] [PubMed]
- Iwatani S, Iijima H, Otake Y, et al. Novel mass spectrometry-based comprehensive lipidomic analysis of plasma from patients with inflammatory bowel disease. J Gastroenterol Hepatol 2020;35:1355-64. [Crossref] [PubMed]
- Pochard C, Gonzales J, Bessard A, et al. PGI2 Inhibits Intestinal Epithelial Permeability and Apoptosis to Alleviate Colitis. Cell Mol Gastroenterol Hepatol 2021;12:1037-60. [Crossref] [PubMed]
- Liu R, Qiao S, Shen W, et al. Disturbance of Fatty Acid Desaturation Mediated by FADS2 in Mesenteric Adipocytes Contributes to Chronic Inflammation of Crohn's Disease. J Crohns Colitis 2020;14:1581-99. [Crossref] [PubMed]
- Sasaki M, Johtatsu T, Kurihara M, et al. Energy metabolism in Japanese patients with Crohn's disease. J Clin Biochem Nutr 2010;46:68-72. [Crossref] [PubMed]
- Barot LR, Rombeau JL, Feurer ID, et al. Caloric requirements in patients with inflammatory bowel disease. Ann Surg 1982;195:214-8. [Crossref] [PubMed]
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19 Suppl A:5A-36A.
- Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505-12. [Crossref] [PubMed]
- Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439-44. [Crossref] [PubMed]
- Fan F, Mundra PA, Fang L, et al. Lipidomic Profiling in Inflammatory Bowel Disease: Comparison Between Ulcerative Colitis and Crohn's Disease. Inflamm Bowel Dis 2015;21:1511-8. [Crossref] [PubMed]
- Scoville EA, Allaman MM, Brown CT, et al. Alterations in Lipid, Amino Acid, and Energy Metabolism Distinguish Crohn's Disease from Ulcerative Colitis and Control Subjects by Serum Metabolomic Profiling. Metabolomics 2018;14:17. [Crossref] [PubMed]
- Murgia A, Hinz C, Liggi S, et al. Italian cohort of patients affected by inflammatory bowel disease is characterised by variation in glycerophospholipid, free fatty acids and amino acid levels. Metabolomics 2018;14:140. [Crossref] [PubMed]
- Nahar L, Onder A, Sarker SD. A review on the recent advances in HPLC, UHPLC and UPLC analyses of naturally occurring cannabinoids (2010-2019). Phytochem Anal 2020;31:413-57. [Crossref] [PubMed]
- Rodriguez-Aller M, Gurny R, Veuthey JL, et al. Coupling ultra high-pressure liquid chromatography with mass spectrometry: constraints and possible applications. J Chromatogr A 2013;1292:2-18. [Crossref] [PubMed]
- Zhang X, Song L, Li L, et al. Phosphatidylserine externalized on the colonic capillaries as a novel pharmacological target for IBD therapy. Signal Transduct Target Ther 2021;6:235. [Crossref] [PubMed]
- He Z, Si Y, Jiang T, et al. Phosphotidylserine exposure and neutrophil extracellular traps enhance procoagulant activity in patients with inflammatory bowel disease. Thromb Haemost 2016;115:738-51. [Crossref] [PubMed]
- Wu SE, Hashimoto-Hill S, Woo V, et al. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature 2020;586:108-12. [Crossref] [PubMed]
- Zhang X, Choi FF, Zhou Y, et al. Metabolite profiling of plasma and urine from rats with TNBS-induced acute colitis using UPLC-ESI-QTOF-MS-based metabonomics--a pilot study. FEBS J 2012;279:2322-38. [Crossref] [PubMed]
- Donowitz M. Arachidonic acid metabolites and their role in inflammatory bowel disease. An update requiring addition of a pathway. Gastroenterology 1985;88:580-7. [Crossref] [PubMed]
- Marion-Letellier R, Savoye G, Beck PL, et al. Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm Bowel Dis 2013;19:650-61. [Crossref] [PubMed]
- Dogay Us G, Mushtaq S. N-3 fatty acid supplementation mediates lipid profile, including small dense LDL, when combined with statins: a randomized double blind placebo controlled trial. Lipids Health Dis 2022;21:84. [Crossref] [PubMed]
- Xiong Q, Zhao J, Tian C, et al. Regulation of a High-Iron Diet on Lipid Metabolism and Gut Microbiota in Mice. Animals (Basel) 2022;12:2063. [Crossref] [PubMed]
- Han H, Wang M, Zhong R, et al. Depletion of Gut Microbiota Inhibits Hepatic Lipid Accumulation in High-Fat Diet-Fed Mice. Int J Mol Sci 2022;23:9350. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


