Development and validation of a predictive model for in-hospital mortality in patients with sepsis-associated liver injury
Introduction
Sepsis is a major disease in intensive care units (ICU) worldwide, with high rates of morbidity and mortality (1). According to the International Surviving Sepsis Campaign, sepsis is defined as life-threatening organ dysfunction caused by the host response to infection disorders and is characterized by systemic inflammation that is commonly associated with organ dysfunction or failure (2). The liver is the central organ of the body in terms of defense against exogenous infection, with a large collection of phagocytic cells present. Also, the liver sinusoids are rich in various types of lymphocytes, which enable the identification and capture of antigens from the gastrointestinal tract and blood. Moreover, the liver is an important site of adaptive immunity (3,4), and is intimately related to the maintenance of the microbial barrier of the gastrointestinal tract. Additionally, it can also secrete bile acids and regulate the intestinal flora through the hepato-enteric axis (5). In infectious diseases, after the intestinal-epithelial barrier, the liver constitutes the second line of defense to clear invading bacteria and bacterial products and limit the further spread of bacteria into the body. During sepsis, the release of a large number of inflammatory factors inhibits the expression of tight junction proteins and upregulates intestinal epithelial cell apoptosis, resulting in increased permeability of the intestinal barrier. A large number of intestinal microbes enter the blood and the liver through the portal vein, which directly aggravates liver injury (6). In addition, a vigorous immunogenic response within the liver, while contributing to the clearance of microbial products, may also lead to liver injury due to overwhelming systemic inflammatory responses (7).
Sepsis-associated liver injury (SALI) is a result of the combined effects of multiple mechanisms, including the systemic inflammatory response, immune dysregulation, and microcirculatory disturbance (8). SALI primarily manifests in two forms: hypoxic hepatitis (HH) and cholestasis (9). HH is often accompanied by circulatory and respiratory failure, and acute heart failure and septic shock are the most common predisposing factors (10). The occurrence of cholestasis is closely related to the dysfunction and inflammation of liver and bile duct cells caused by proinflammatory cytokines and inflammatory mediators. Impaired cells lead to the down-regulation of the transport system function and the normal operation of bile is impaired, which leads to cholestasis flow (11).
Sepsis is one of the leading causes of HH and cholestasis in the ICU. Two multicenter studies of acquired HH in the ICU reported that 32% and 33% of patients had HH combined with septic shock, respectively (12,13), while the proportion of cholestasis triggered by septic shock was approximately 28–33% (3,14). At present, there is a lack of clear diagnostic criteria for SALI. The incidence of SALI varies according to different diagnostic criteria; Kobashi et al. reported an incidence rate of 34.7% (15). Severe sepsis can lead to a high mortality rate in intensive care units due to a variety of factors, including multiple visceral damage and dysfunction due to inflammation that has not been controlled, multidrug-resistant bacterial infections, and ineffective anti-infection treatments (2). Besides the risks associated with sepsis, liver injury poses a number of additional risks, including comorbid organ injury in synergy with the liver, increased drug toxicity as a result of decreased liver biotransformation function, which may result in a higher death rate. Without exception, all of the studies on HH, cholestasis, or SALI have reported extremely high mortality rates (up to 53–61.5%) (12,13,16). Given the severe adverse consequences for SALI patients, the early identification of patients with a high risk of death can help clinicians optimize the clinical diagnosis and treatment strategies, which is crucial to improving the prognosis of patients. To date, prediction models have been applied to predict the mortality risk of sepsis patients. Prediction models based on the clinical data of the patients were developed by Ren and Zeng to assess the in-hospital mortality of sepsis patients and the 90-day mortality of those patients. Previously, prediction models were designed to cover the entire spectrum of sepsis patients, whereas the models may not be applicable to this particular group of patients in SALI (17,18). At present, there are few studies on SALI and no short-term predictive models for SALI patients. Furthermore, there are currently no large-scale studies or accurate short-term predictive models for SALI patients. The present study aims to address this. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4319/rc).
Methods
Data source
Data for this retrospective study were obtained from the Medical Information Mart for Intensive Care (MIMIC)-IV database (Version 1.0). This public database is maintained by the Computational Physiology Laboratory of the Massachusetts Institute of Technology (Massachusetts Institute of Technology, Cambridge, Massachusetts, USA), and includes the clinical information of patients admitted to the ICU of the Beth Israel Women‘s Medical Center (BIDMC, Boston, Massachusetts, USA) from 2008 to 2019 (19).
The included patients were de-identified according to the Health Insurance Portability and Accountability Act (HIPAA). Since the current project does not affect clinical nursing, the requirement for individual patient consent was waived. Members of the team (Run Sun) were given access to the database and were responsible for the data extraction (CITE number: 45997657).
Patient population
The inclusion criteria were as follows: (I) patients with sepsis; (II) patients age ≥18; (III) patients who remained in the ICU for at least 48 hours; and (IV) patients who met the SALI diagnostic criteria at least once. Sepsis was defined according to the Sepsis3.0 guidelines. The first record was selected for patients with multiple admissions to the ICU during hospitalization.
The exclusion criteria were as follows (20-23): (I) patients without liver injury; (II) those with primary liver diseases, such as acute or chronic viral hepatitis, cirrhosis and chronic liver diseases, acute or chronic liver failure, and liver necrosis; (III) patients with liver trauma; (IV) those with autoimmune hepatitis; (V) patients with hepatic vascular infarction; (VI) patients with toxic hepatitis; (VII) those with cholangitis and acute cholecystitis with biliary obstruction; (VIII) patients with bile duct obstruction; (IX) patients with bile duct and ampullary tumors (Table S1); (X) length of ICU stay <48 hours.
SALI
There are currently no definite diagnostic criteria for SALI. Based on previous studies, SALI patients included in this study were further divided into HH and cholestasis groups. HH was defined as a 10-fold increase in the levels of alanine aminotransferase or aspartate aminotransferase above the upper limit of normal (400 U/dL) (20), and the cholestasis group was defined as serum bilirubin >2 mg/dL (21,23). The time that the patient entered the ICU was set as 0 h, and the time of the first recording of SALI was defined as the liver injury occurrence time.
Data extraction and management
Data were extracted in a structured query language (SQL) using Navicat Premium version 15.0.12 (PremiumSoft CyberTech Ltd, Hongkong), and codes in the database were all available on GitHub (https://github.com/MIT-LCp/mimic-code). In order to facilitate the practical application and generalization of predictive models, variable extraction is considered on the basis of early acquisition and easy acquisition. We retrospectively collected the following data: (I) demographic data, including age and gender; (II) type and occurrence time of liver injury; (III) chronic complications: chronic complications were defined according to International Classification of Diseases 9 (ICD) and ICD-10, and included hypertension, hyperlipidemia, diabetes both with and without complication, atrial fibrillation, old myocardial infarction, chronic heart failure, chronic pulmonary disease, chronic renal disease, peripheral vascular disease, cerebrovascular disease, malignant cancer, and hypothyroidism; (IV) septic shock, anemia after acute hemorrhage, and acute organ injury; (V) infection site and pathogenic microorganism; (VI) vital signs and urine output within 24 hours of hospitalization in the ICU, in which the vital signs were expressed as the mean; (VII) the first laboratory examination data after ICU hospitalization; (VIII) supportive treatments including mechanical ventilation and renal replacement therapy; (IX) drugs and other treatments, such as blood transfusion, vasoactive drugs, cardiovascular drugs, frozen plasma, extraintestinal nutrition, and anticoagulants; and (V) the first Simplified Acute Physiology Score2 (SAPS2) and sequential organ failure assessment (SOFA) score in the ICU.
The determination of infection site and pathogenic microorganism culture results were lagging, so it was not applicable to incorporate them into the predictive model. The SOFA and SAPS2 scores are commonly used in ICU to evaluate severity and patient prognosis, which were used for comparison with the performance of the predictive model in this study. Continuous variables with more than 20% missingness were removed, and the remaining missing data were filled by interpolation (Table S2, Figure S1).
Statistical analysis
The Shapiro-Wilk test was applied to evaluate whether the samples conformed to a normal distribution. Normally distributed continuous variables were expressed as the mean ± standard deviation (SD), while those that did not conform to a normal distribution were expressed as the median (interquartile range, IQR). Classified variables were expressed as frequency and percentage. The non-parametric test (Mann-Whitney U test) was used to analyze data with non-normal distribution or heterogeneity of variance, and the Pearson chi-square test was used to classify the variables.
Feature selection and model development
In the clinic, SAPS II and SOFA scores are commonly used for assessing patient outcomes, and their inclusion as variables in predictive models will undoubtedly enhance the accuracy of the models. In actual clinical work, obtaining the exact score depends on the completeness of information regarding its composition, and including the score in model building can actually decrease its usefulness. As a result, in this study, the score was not used in variable screening and model construction, but was used to contrast with the model. To remove redundant variants, the least absolute shrinkage and selection operator (LASSO) regression was applied to feature the selection of prognostic-related variables. Multivariate logistic regression analysis was performed on the variable combinations corresponding to the minimum mean square error (MMSE), and independent factors with statistically significant differences were used to construct the predictive model. Patients were randomly assigned to a training (80%) or validation (20%) cohort, and nomograms were developed based on the training cohort to visualize the model. The predicted outcome was the patient’s risk of in-hospital mortality. To evaluate the performance of the model, the area under the curve (AUC) and bootstrap resampling methods (1,000 iterations) were applied. Also, the clinical practicability and net profit of the predictive model were evaluated by Decision Curve Analysis (DCA). IBM SPSS Statistics version 26.0 (SPSS Inc., Chicago, IL, USA, 2019) was used for data analysis and (R Foundation for Statistical Computing, Vienna, Austria) was utilized for model construction and validation. A two-sided P value smaller than 0.05 suggested statistical significance. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Baseline characteristics of the survival and non-survival groups
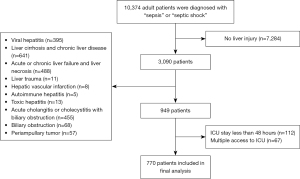
Figure 1 shows the detailed patient recruitment process. Based on the inclusion and exclusion criteria, 770 patients were included in the final analysis.
The baseline characteristics of the survival and non-survival groups are shown in Table 1. The survival group had a longer hospitalization time but there was no significant difference in the ICU hospitalization time between the two groups. In addition, the non-survival group had a higher proportion of liver injury in the form of HH, and liver injury occurred later. In terms of chronic complications, the non-survival group had a higher proportion of diabetes without complications, atrial fibrillation, chronic heart failure, chronic pulmonary disease, peripheral vascular disease, and cancer. Acute organ injury was more common in the non-survival group, with a higher proportion of acute heart failure, acute myocardial infrastructure, acute respiratory failure, and coagulation disorder.
Table 1
| Variables | All (n=770) | Survival group (n=435) | Non-survival group (n=335) | P value |
|---|---|---|---|---|
| Hospital stay (days) | ||||
| LOS-hospital | 19.7 [10.2, 33.0] | 22.0 [12.8, 34.6] | 15.1 [7.0, 30.1] | <0.001 |
| LOS-ICU | 8.5 [4.3, 16.1] | 8.5 [4.2, 16.3] | 8.5 [4.5, 16.1] | 0.998 |
| Liver injury | ||||
| Type, n (%) | ||||
| Cholestasis | 652 (84.7) | 387 (89.0) | 265 (79.1) | <0.001 |
| Hypoxia | 118 (15.3) | 48 (11.0) | 70 (20.9) | |
| Occurrence time (hours) | 20.0 [4.0, 73.0] | 12.0 [2.0, 51.0] | 32.0 [8.0, 95.0] | <0.001 |
| Demography | ||||
| Age (years) | 67.1 [56.9, 76.5] | 65.1 [54.3, 74.7] | 68.2 [60.0, 78.5] | <0.001 |
| Gender, n (%) | ||||
| Female | 324 (42.1) | 176 (40.5) | 148 (44.2) | 0.3 |
| Male | 446 (57.9) | 259 (59.5) | 187 (55.8) | |
| Comorbidity, n (%) | ||||
| Hypertension | 495 (64.3) | 275 (63.2) | 220 (65.7) | 0.481 |
| Hyperlipidemia | 238 (30.9) | 131 (30.1) | 107 (31.9) | 0.587 |
| Diabetes without complication | 190 (24.7) | 95 (21.8) | 95 (28.4) | 0.038 |
| Diabetes with complication | 75 (9.7) | 36 (8.3) | 39 (11.6) | 0.118 |
| Atrial fibrillation | 288 (37.4) | 137 (31.5) | 151 (45.1) | <0.001 |
| Old myocardial infarction | 64 (8.3) | 33 (7.6) | 31 (9.3) | 0.406 |
| Chronic heart failure | 209 (27.1) | 102 (23.4) | 107 (31.9) | 0.009 |
| Chronic pulmonary disease | 209 (27.1) | 99 (22.8) | 110 (32.8) | 0.002 |
| Chronic renal disease | 212 (27.5) | 111 (25.5) | 101 (30.1) | 0.154 |
| Peripheral vascular disease | 114 (14.8) | 54 (12.4) | 60 (17.9) | 0.033 |
| Cerebrovascular disease | 78 (10.1) | 36 (8.3) | 42 (12.5) | 0.052 |
| Malignant cancer | 122 (15.8) | 56 (12.9) | 66 (19.7) | 0.01 |
| Hypothyroidism | 108 (14.0) | 55 (12.6) | 53 (15.8) | 0.208 |
| Acute organ injury, n (%) | ||||
| Acute heart failure | 207 (26.9) | 97 (22.3) | 110 (32.8) | 0.001 |
| Septic shock | 543 (70.5) | 296 (68.0) | 247 (73.7) | 0.086 |
| Acute myocardial infarction | 87 (11.3) | 29 (6.7) | 58 (17.3) | <0.001 |
| Acute respiratory failure | 471 (61.2) | 238 (54.7) | 233 (69.6) | <0.001 |
| Acute renal failure | 547 (71.0) | 297 (68.7) | 250 (74.6) | 0.054 |
| Coagulation disorder | 288 (37.4) | 144 (33.1) | 144 (43.0) | 0.005 |
| Acute post-hemorrhagic anemia | 170 (22.1) | 91 (20.9) | 79 (23.6) | 0.377 |
| Toxic encephalopathy | 100 (13.0) | 50 (11.5) | 50 (14.9) | 0.16 |
Continuous variables in the table did not follow a normal distribution and are expressed as median [interquartile range] and categorical variables as frequencies (percentages). LOS, length of stay; ICU, intensive care unit.
The microbial results and infection site characteristics of the two groups are shown in Table 2. The five pathogens with the highest detection rate were as follows: Escherichia coli, Methicillin-susceptible staphylococcus, other gram-negative bacteria, Streptococcus and Candida. The proportion of Escherichia coli in the non-survival group was lower than that in the survival group, and there were no significant differences in the proportions of the other pathogenic microorganisms between the two groups. The most common site of infection was the lung (pulmonary infection). The non-survival group had more pulmonary infections and fewer abdominal infections compared to the survival group.
Table 2
| Variables | All (n=770) | Survival group (n=435) | Non-survival group (n=335) | P value |
|---|---|---|---|---|
| Pathogenic microorganism, n (%) | ||||
| Escherichia coli | 87 (11.3) | 62 (14.3) | 25 (7.5) | 0.003 |
| Streptococcus | 70 (9.1) | 47 (10.8) | 23 (6.9) | 0.059 |
| Methicillin-susceptible staphylococcus | 87 (11.3) | 52 (12.0) | 35 (10.4) | 0.513 |
| Other gram-negative bacteria | 78 (10.1) | 50 (11.5) | 28 (8.4) | 0.153 |
| Methicillin-resistant staphylococcus | 51 (6.6) | 25 (5.7) | 26 (7.8) | 0.265 |
| Pseudomonad | 50 (6.5) | 27 (6.2) | 23 (6.9) | 0.713 |
| Candida | 54 (7.0) | 34 (7.8) | 20 (6.0) | 0.32 |
| Klebsiella pneumoniae | 41 (5.3) | 25 (5.7) | 16 (4.8) | 0.552 |
| Enterococcus | 17 (2.2) | 13 (3.0) | 4 (1.2) | 0.093 |
| Other staphylococci | 32 (4.2) | 18 (4.1) | 14 (4.2) | 0.977 |
| Infection site, n (%) | ||||
| Lung | 374 (48.6) | 188 (43.2) | 186 (55.5) | <0.001 |
| Subcutaneous skin and soft tissue | 219 (28.4) | 117 (26.9) | 102 (30.4) | 0.279 |
| Urinary tract | 158 (20.5) | 97 (22.3) | 61 (18.2) | 0.164 |
| Abdominal cavity | 177 (23.0) | 112 (25.7) | 65 (19.4) | 0.038 |
| Catheter correlation | 145 (18.8) | 86 (19.8) | 59 (17.6) | 0.448 |
The characteristics of the two groups during ICU hospitalization are shown in Table 3. In terms of vital characteristics, there were no significant differences in heart rate and blood pressure between the two groups, but the non-survival group had a lower body temperature and 24-hour urine output. Laboratory examinations revealed that the non-survival group had lower red blood cell, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and total bilirubin levels, and higher levels of RDW, lactate dehydrogenase, blood urea nitrogen (BUN), partial thromboplastin time (PTT), and bicarbonate. Moreover, the proportion of non-survivors who required supportive treatment was higher, and more patients in this group underwent invasive ventilation and renal replacement therapy. In terms of treatment, a higher proportion of patients in the non-survival group received blood transfusion, vasoactive drugs, and frozen plasma infusion. As for the disease scores, patients in the non-survival group had higher SAPS2 scores but there were no significant differences in the SOFA scores between the two groups (P>0.05).
Table 3
| Variables | All (n=770) | Survival group (n=435) | Non-survival group (n=335) | P value |
|---|---|---|---|---|
| Vital sign | ||||
| Heart rate (bpm) | 96.0 [84.0, 108.0] | 97.0 [84.0, 109.0] | 96.0 [83.0, 107.0] | 0.743 |
| Systolic pressure (mmHg) | 106.0 [99.0, 114.0] | 106.0 [99.0, 113.0] | 105.0 [99.0, 116.0] | 0.764 |
| Diastolic pressure (mmHg) | 58.0 [53.0, 65.0] | 59.0 [54.0, 65.0] | 58.0 [52.0, 65.0] | 0.119 |
| Mean arterial pressure (mmHg) | 72.0 [67.0, 78.0] | 73.0 [68.0, 78.0] | 71.0 [66.0, 78.0] | 0.098 |
| Respiratory rate (bpm) | 22.0 [18.0, 25.0] | 22.0 [19.0, 25.0] | 22.0 [18.0, 25.0] | 0.033 |
| Temperature (℃) | 36.90 [36.58, 37.40] | 37.04 [36.69, 37.51] | 36.76 [36.48, 37.18] | <0.001 |
| SPO2 (%) | 97.1 [95.8, 98.5] | 97.1 [95.7, 98.5] | 97.1 [95.8, 98.7] | 0.62 |
| Urine output (mL/24 h) | 1,195 [615, 1,980] | 1,378 [817, 2,200] | 870 [380, 1675] | <0.001 |
| Laboratory results | ||||
| Red blood cell (m/UL) | 3.3 [2.8, 3.9] | 3.4 [2.9, 4.0] | 3.2 [2.7, 3.8] | 0.017 |
| Hemoglobin (g/dL) | 9.8 [8.3, 11.4] | 10.2 [8.4, 11.8] | 9.5 [8.1, 11.1] | 0.011 |
| Hematocrit (%) | 30.3 [25.7, 35.1] | 30.8 [26.1, 35.8] | 29.6 [25.3, 34.3] | 0.123 |
| MCV (fl) | 91.0 [87.0, 96.0] | 91.0 [87.0, 95.0] | 92.0 [87.0, 98.0] | 0.005 |
| MCH (pg) | 30.0 [28.5, 31.4] | 29.9 [28.3, 31.3] | 30.2 [28.6, 31.4] | 0.275 |
| MCHC (g/dL) | 32.6 [31.5, 33.7] | 32.8 [31.6, 33.8] | 32.3 [31.3, 33.5] | <0.001 |
| RDW (%) | 15.7 [14.3, 17.8] | 15.3 [13.9, 17.2] | 16.5 [14.8, 18.8] | <0.001 |
| Platelet (K/UL) | 170.0 [98.0, 247.0] | 179.0 [104.0, 249.0] | 156.0 [94.0, 239.0] | 0.099 |
| White blood cell (K/UL) | 12.7 [7.3, 19.5] | 13.0 [7.3, 20.0] | 12.4 [7.4, 19.0] | 0.613 |
| Neutrophils (%) | 81.6 [71.2, 87.7] | 80.5 [71.0, 87.270] | 82.1 [72.5, 88.0] | 0.114 |
| Monocytes (%) | 4.0 [2.4, 6.8] | 4.0 [2.5, 6.4] | 4.0 [2.3, 7.0] | 0.981 |
| Lymphocytes (%) | 7.0 [3.9, 11.0] | 7.150 [4.0, 11.0] | 6.6 [3.7, 11.0] | 0.528 |
| Eosinophils (%) | 0.1 [0.0, 1.0] | 0.1 [0.0, 1.0] | 0.05 [0.0, 1.0] | 0.416 |
| Basophils (%) | 0.0 [0.0, 0.2] | 0.0 [0.0, 0.2] | 0.0 [0.0, 0.2] | 0.727 |
| Albumin (g/dL) | 2.6 [2.2, 3.0] | 2.6 [2.2, 3.0] | 2.5 [2.2, 2.9] | 0.214 |
| ALT (IU/L) | 36.0 [20.0, 78.0] | 37.0 [21.0, 84.0] | 35.0 [19.0, 75.0] | 0.358 |
| AST (IU/L) | 58.0 [33.0, 132.0] | 59.0 [33.0, 123.0] | 55.0 [33.0, 144.0] | 0.765 |
| ALP (IU/L) | 100.0 [64.0, 174.0] | 99.0 [63.0, 166.0] | 102.0 [65.0, 183.0] | 0.194 |
| Total bilirubin (mg/dL) | 2.1 [1.0, 3.0] | 2.2 [1.1, 3.2] | 1.6 [0.7, 2.9] | <0.001 |
| LDH (IU/L) | 358.0 [249.0, 572.0] | 323.0 [237.0, 513.0] | 415.0 [282.0, 695.0] | <0.001 |
| BUN (mg/dL) | 31.0 [19.0, 52.0] | 29.0 [18.0, 48.0] | 35.0 [21.0, 54.0] | 0.008 |
| Creatinine (mg/dL) | 1.4 [0.9, 2.5] | 1.4 [0.9, 2.4] | 1.4 [0.9, 2.7] | 0.652 |
| Glucose (mg/dL) | 131.0 [104.0, 170.0] | 129.0 [105.0, 161.0] | 137.0 [103.0, 176.0] | 0.324 |
| Potassium (mEq/L) | 4.1 [3.7, 4.6] | 4.1 [3.7, 4.5] | 4.1 [3.7, 4.7] | 0.473 |
| Sodium (mEq/L) | 137.0 [134.0, 141.0] | 137.0 [134.0, 141.0] | 137.0 [134.0, 141.0] | 0.247 |
| Chloride (mEq/L) | 103.0 [99.0, 108.0] | 104.0 [99.0, 109.0] | 103.0 [99.0, 108.0] | 0.642 |
| Calcium (mg/dL) | 7.9 [7.3, 8.5] | 7.9 [7.3, 8.4] | 8.0 [7.4, 8.5] | 0.053 |
| PT (s) | 16.2 [14.0, 19.6] | 16.2 [14.1, 18.9] | 16.3 [13.8, 20.8] | 0.488 |
| PTT (s) | 34.3 [30.0, 44.6] | 33.1 [29.4, 42.1] | 36.4 [31.2, 50.2] | <0.001 |
| INR | 1.5 [1.3, 1.8] | 1.5 [1.3, 1.7] | 1.5 [1.3, 1.9] | 0.472 |
| PO2 (mmHg) | 85.0 [49.0, 154.0] | 88.0 [50.0, 161.0] | 81.0 [47.0, 139.0] | 0.276 |
| PCO2 (mmHg) | 39.0 [33.0, 47.0] | 39.0 [33.0, 45.0] | 40.0 [33.0, 48.0] | 0.133 |
| PH | 7.35 [7.28, 7.41] | 7.35 [7.28, 7.41] | 7.35 [7.27, 7.42] | 0.821 |
| Total CO2 (mEq/L) | 23.0 [19.0, 26.0] | 22.0 [19.0, 26.0] | 23.0 [20.0, 27.0] | 0.162 |
| Base excess (mEq/L) | −3.0 [−7.0, 0.0] | −3.0 [−7.0, 0.0] | −3.0 [−7.0, 0.0] | 0.468 |
| Bicarbonate (mEq/L) | 21.0 [18.0, 24.0] | 20.0 [17.0, 23.0] | 21.0 [18.0, 24.0] | 0.034 |
| Anion gap (mEq/L) | 16.0 [13.0, 19.0] | 16.0 [13.0, 19.0] | 16.0 [13.0, 19.0] | 0.335 |
| Lactate (mmol/L) | 2.3 [1.5, 3.5] | 2.2 [1.5, 3.5] | 2.3 [1.5, 3.6] | 0.349 |
| Treatment, n (%) | ||||
| Invasive ventilation | 584 (75.844) | 311 (71.494) | 273 (81.493) | 0.001 |
| Renal replacement therapy | 246 (31.948) | 106 (24.368) | 140 (41.791) | <0.001 |
| Blood transfusion | 494 (64.156) | 264 (60.690) | 230 (68.657) | 0.022 |
| Vasoactive drugs | 662 (85.974) | 361 (82.989) | 301 (89.851) | 0.007 |
| Cardiotonic drugs | 73 (9.481) | 35 (8.046) | 38 (11.343) | 0.122 |
| Frozen plasma | 257 (33.377) | 125 (28.736) | 132 (39.403) | 0.002 |
| Extraintestinal nutrition | 158 (20.519) | 85 (19.540) | 73 (21.791) | 0.443 |
| Anticoagulant | 115 (14.935) | 59 (13.563) | 56 (16.716) | 0.224 |
| Severity score | ||||
| SOFA | 9.0 [6.0, 11.0] | 9.0 [6.0, 11.0] | 9.0 [6.0, 12.0] | 0.358 |
| SAPSII | 48.0 [39.0, 58.0] | 45.0 [36.0, 54.0] | 51.0 [43.0, 60.0] | <0.001 |
Continuous variables in the table did not follow a normal distribution and are expressed as median [interquartile range] and categorical variables as frequencies (percentages). ICU, intensive care unit; SPO2, saturation of pulse oxygen; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio; PO2, oxygen partial pressure; PCO2, carbon dioxide partial pressure; PH, potential of hydrogen; SAPS2, simplified acute physiology score II (SAPSII); SOFA, sequential organ failure assessment score.
Feature selection
The 79 variables related to prognosis were screened by LASSO regression and 10-fold cross-validation (Figure 2A). In the minimum mean square errors (λ=0.038), the number of features was reduced to 11 (Figure 2B), including age, SALI type, occurrence time, atrial fibrillation acute respiratory failure, acute myocardial infarction, temperature, urine output, RDW, PTT, and renal replacement therapy. The regression coefficients for variables in the LASSO regression were presented in Table S3.

Multivariable logistic analysis
Multivariate logistic analysis was performed to analyze the 11 potential predictors (Table 4). The results showed that SALI type, occurrence time, acute respiratory failure, acute myocardial infarction, temperature, urine output, RDW, PTT, and renal replacement therapy were independent prognostic factors for SALI patients (P<0.05).
Table 4
| Variables | OR | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age | 1.006 | 0.994 | 1.018 | 0.365 |
| Type | 1.653 | 1.048 | 2.617 | 0.031 |
| Occurrence time | 1.006 | 1.004 | 1.009 | <0.001 |
| Atrial fibrillation | 1.425 | 1.000 | 2.032 | 0.05 |
| Acute respiratory failure | 1.781 | 1.269 | 2.509 | 0.001 |
| Acute myocardial infarction | 2.703 | 1.617 | 4.603 | <0.001 |
| Temperature | 0.667 | 0.512 | 0.863 | 0.002 |
| Urine output | 1.000 | 1.000 | 1.000 | 0.003 |
| RDW | 1.167 | 1.103 | 1.238 | <0.001 |
| PTT | 1.007 | 1.001 | 1.014 | 0.035 |
| Renal replacement therapy | 1.441 | 1.001 | 2.073 | 0.049 |
OR, odds ratio; CI, confidence interval; RDW, red blood cell distribution width; PTT, partial thromboplastin time.
Nomogram development
Nine independent variables were used to develop a model to predict the in-hospital mortality of SALI patients, which was presented in the form of a visual nomogram (Figure 3). By establishing the hazard ratios of these risk factors, the prognosis of each patient can be scored, and the sum of scores of each variable can be used to evaluate the risk of in-hospital mortality of SALI patients.

Discrimination and calibration
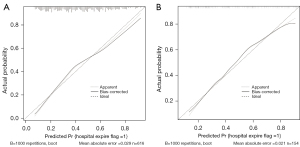
The AUC of the prediction model in the training and validation cohorts were 0.753 (95% CI: 0.715–0.791) and 0.783 (95% CI: 0.749–0.817), respectively, which were significantly better than the SOFA score (AUC of the training cohort: 0.516, 95% CI: 0.47–0.563; AUC of the validation cohort: 0.531, 95% CI: 0.438–0.624) and SAPS2 score (AUC of the training cohort: 0.612, 95% CI: 0.567–0.657; AUC of the validation cohort: 0.629, 95% CI: 0.537–0.718). Compared with the SOFA and SAPS2 scores, the predictive model showed better discrimination in predicting the risk of in-hospital death of SALI patients (Figure 4). The specificity and sensitivity of the predictive model were 0.704 and 0.698, respectively. The 1,000 bootstrap method was adopted to calibrate the model. In both cohorts, the calibration curves were slightly deviated, but good consistency between observation and prediction was still observed, with Brier scores of 0.199 and 0.188, respectively (Figure 5).

Clinical utility
DCA was applied to evaluate the clinical utility of the predictive model and scoring methods. In the training cohort, when the probability threshold (PT) was greater than 0.1, the medical intervention guided by the nomogram was found to provide a superior net benefit than the SOFA and SAPS2 scores (Figure 6A). In the validation cohort, when the PT was greater than 0.1, the treatment guided by the nomogram could provide a higher net benefit than the SOFA and SAPS2 scores (Figure 6B).

Discussion
According to our results, 29.8% of sepsis patients had a liver injury (3,090 of 10,374 patients), but after strict exclusion of other factors that could cause liver dysfunction, the incidence of SALI decreased to 9.15 %, which was lower than that reported by Kobashi et al. and Saini et al. This discrepancy was due to the different diagnostic criteria for hypoxic injury; the inclusion criteria for hypoxic injury used in Kobashi et al. were alanine aminotransferase (ALT), aspartate aminotransferase (AST), and G-glutamyl transferase (GGT) exceeding the upper limit of normal values, while that applied in Saini et al. was a two-fold exceeding of the upper limit of ALT (15,24). One of the characteristics of HH is the massive and transient increase in serum transaminase activity caused by hypoxic necrosis of hepatocytes in the centrilobular region. The standard chosen in multiple studies of HH in the ICU involves ALT or AST levels exceeding the upper limit of normal value by 10–20-fold (25-29). In this study, the inclusion criterion of the HH group was a >10-fold increase to reflect this unique liver injury. Without exception, and consistent with most studies on SALI, HH, and cholestasis, the present study also observed a high mortality rate; the overall in-hospital mortality rate of SALI patients was 43.5%, and the mortality rate in the HH group was remarkably high (up to 59.3%). However, there are currently few studies on the prognostic aspects of SALI patients, and the development of a predictive model that can effectively provide an early prediction of a patient’s risk is helpful for clinicians to comprehensively assess the true risk of death and offer a clear statement of the condition to families of SALI patients.
The SOFA and SAPS2 scores are the most commonly used disease scores in clinical practice and are widely applied in internal medicine wards and the ICU for predicting the short-term mortality of patients with sepsis (30,31). Surprisingly, these two scores did not show good performance in the prognosis of SALI patients, with the SOFA score showing worse efficacy, indicating that these two scores may not apply to the prognostic prediction of this special group of sepsis patients. In our prediction model, body temperature plays an important role. Higher body temperature was found to be associated with improved prognosis in this study. As reported by Young et al., the early peak temperature in infected patients was associated with a reduced risk of in-hospital mortality, even if the peak temperature exceeded 40.0 ℃ (32). Similar results were also observed by Lee et al. in a prospective study, which showed that higher body temperature was associated with better clinical outcomes (33). Fever is the body’s protective response to infection. In infectious diseases, endogenous and exogenous heat sources lead to an increased body temperature, which is the response of the body against exogenous microorganisms (34). It has also been found that hypothermia increases the occurrence of adverse outcomes in septic patients, which is consistent with our findings (35).
Another important factor is the liver injury occurrence time. Two studies have suggested that the mortality rate of patients with prolonged liver injury is higher, but no other studies have explored the relationship between the liver injury occurrence time and the prognosis of patients (12,27). To facilitate the liver injury occurrence time calculation, we set the time at which patients entered the ICU as 0 hour, and found that compared with the survival group, the time of liver injury in the non-survival group was later. We speculate that the earlier liver dysfunction is triggered by an inflammatory response and hepatic microcirculation disturbance under infection, which can be restored after infection control. Later liver dysfunction may be one of the manifestations of systemic organ failure caused by severe sepsis or may be a concurrent result of other organ injuries. Animal experiments have shown that acute kidney injury (AKI) can trigger liver inflammation by activating kappa B (NF-κF) (36). In addition, acidosis and drug metabolism disorders caused by renal injury can directly or indirectly induce liver injury, increasing the mortality of patients (37). Meanwhile, a subset of patients experiences multiple injuries as the liver injury disease progresses; Jäger et al. reported that new onset jaundice in patients with HH increased the mortality of patients (38). However, more studies are needed to discover the reasons for this discrepancy; further clarifying the existence and causes of this difference by imaging examination or other standards will facilitate a more accurate determination of the liver injury occurrence time and allow for contrasting the differences in the occurrence time of different types of liver injury.
Another more important factor is RDW, which was originally used as a diagnostic indicator of anemia. At present, it is believed that excessive oxidative stress and inflammatory response can not only induce the premature death of red blood cells but also inhibit the maturation of red blood cells, resulting in the premature release of red blood cells into the circulation, ultimately leading to increased RDW, which is suggestive of red blood cell heterogeneity (39). In this study, we found that higher RDW was associated with adverse outcomes. Similarly, a previous study has confirmed the role of RDW in the prognosis of patients with sepsis (40).
Our research has some limitations that should be noted. Firstly, this is a single-center retrospective study, which lacks multi-center participation and external verification, which limits the reliability of research conclusions. Secondly, for the microbial culture information and infection sites that needed to be identified over a long period, we conducted only a preliminary statistical description and did not use this as an alternative predictor, which may have led to some powerful factors not being included in the model. In addition, the imaging examination information is missing. Therefore, in future studies, we will conduct external validation and include some potentially significant factors to further enhance the stability and performance of our model.
Conclusions
In this study, a predictive model for the in-hospital mortality of SALI patients was established, which showed good accuracy and clinical usefulness. This model can help clinicians identify high-risk patients and reduce the incidence of adverse events.
Acknowledgments
Funding: This research received funding from the Natural Science Research Project of Nantong Science and Technology Bureau (Nos. MS12021021, MS12021035); Nantong Health Medicine Research Center; the Jiangsu Planned Projects for Postdoctoral Research Fund (No. 2021K031A); the Deputy General Manager Project of Science and Technology of Jiangsu Province (No. FZ20210652); the Jiangsu Geriatric Clinical Technology Application Research Project (No. LR2021047); Jiangsu Province Elderly Health Research Project (No.LD2021025); and the Science and Technology Research Plan Project of Rugao (No. RG2021SZL001).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4319/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4319/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med 2016;193:259-72. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54-62. [Crossref] [PubMed]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996-1006. [Crossref] [PubMed]
- Islam KB, Fukiya S, Hagio M, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 2011;141:1773-81. [Crossref] [PubMed]
- Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol 2020;72:558-77. [Crossref] [PubMed]
- Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol 2014;33:498-510. [Crossref] [PubMed]
- Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol 2009;36:4-12. [Crossref] [PubMed]
- Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care 2013;19:128-32. [Crossref] [PubMed]
- Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol 2016;4:263-8. [PubMed]
- Jenniskens M, Langouche L, Vanwijngaerden YM, et al. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med 2016;42:16-27. [Crossref] [PubMed]
- Fuhrmann V, Kneidinger N, Herkner H, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med 2009;35:1397-405. [Crossref] [PubMed]
- Raurich JM, Llompart-Pou JA, Ferreruela M, et al. Hypoxic hepatitis in critically ill patients: incidence, etiology and risk factors for mortality. J Anesth 2011;25:50-6. [Crossref] [PubMed]
- Horvatits T, Drolz A, Rutter K, et al. Circulating bile acids predict outcome in critically ill patients. Ann Intensive Care 2017;7:48. [Crossref] [PubMed]
- Kobashi H, Toshimori J, Yamamoto K. Sepsis-associated liver injury: Incidence, classification and the clinical significance. Hepatol Res 2013;43:255-66. [Crossref] [PubMed]
- Whitehead MW, Hainsworth I, Kingham JG. The causes of obvious jaundice in South West Wales: perceptions versus reality. Gut 2001;48:409-13. [Crossref] [PubMed]
- Ren Y, Zhang L, Xu F, et al. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with sepsis and lung infection. BMC Pulm Med 2022;22:17. [Crossref] [PubMed]
- Zeng Q, He L, Zhang N, et al. Prediction of 90-Day Mortality among Sepsis Patients Based on a Nomogram Integrating Diverse Clinical Indices. Biomed Res Int 2021;2021:1023513. [Crossref] [PubMed]
- Johnson A, Bulgarelli L, Pollard T, et al. (2021). MIMIC-IV (version 1.0). PhysioNet. Available online:
10.13026/s6n6-xd98 10.13026/s6n6-xd98 - Lescot T, Karvellas C, Beaussier M, et al. Acquired liver injury in the intensive care unit. Anesthesiology 2012;117:898-904. [Crossref] [PubMed]
- Brienza N, Dalfino L, Cinnella G, et al. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med 2006;32:267-74. [Crossref] [PubMed]
- Lin J, Gu C, Zhang S, et al. Sites and Causes of Infection in Patients with Sepsis-Associated Liver Dysfunction: A Population Study from the Medical Information Mart for Intensive Care III. Med Sci Monit 2021;27:e928928. [Crossref] [PubMed]
- Kramer L, Jordan B, Druml W, et al. Incidence and prognosis of early hepatic dysfunction in critically ill patients--a prospective multicenter study. Crit Care Med 2007;35:1099-104. [Crossref] [PubMed]
- Saini K, Bolia R, Bhat NK. Incidence, predictors and outcome of sepsis-associated liver injury in children: a prospective observational study. Eur J Pediatr 2022;181:1699-707. [Crossref] [PubMed]
- Lee YI, Kang MG, Ko RE, et al. The Impact of Hypoxic Hepatitis on Clinical Outcomes after Extracorporeal Cardiopulmonary Resuscitation. J Clin Med 2020;9:2994. [Crossref] [PubMed]
- Jung C, Fuernau G, Eitel I, et al. Incidence, laboratory detection and prognostic relevance of hypoxic hepatitis in cardiogenic shock. Clin Res Cardiol 2017;106:341-9. [Crossref] [PubMed]
- Van den Broecke A, Van Coile L, Decruyenaere A, et al. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann Intensive Care 2018;8:15. [Crossref] [PubMed]
- Jonsdottir S, Arnardottir MB, Andresson JA, et al. Prevalence, clinical characteristics and outcomes of hypoxic hepatitis in critically ill patients. Scand J Gastroenterol 2022;57:311-8. [Crossref] [PubMed]
- Aboelsoud MM, Javaid AI, Al-Qadi MO, et al. Hypoxic hepatitis - its biochemical profile, causes and risk factors of mortality in critically-ill patients: A cohort study of 565 patients. J Crit Care 2017;41:9-15. [Crossref] [PubMed]
- Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019;23:374. [Crossref] [PubMed]
- Hu T, Lv H, Jiang Y. The association between four scoring systems and 30-day mortality among intensive care patients with sepsis: a cohort study. Sci Rep 2021;11:11214. [Crossref] [PubMed]
- Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med 2012; [Crossref] [PubMed]
- Lee BH, Inui D, Suh GY, et al. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care 2012;16:R33. [Crossref] [PubMed]
- Walter EJ, Hanna-Jumma S, Carraretto M, et al. The pathophysiological basis and consequences of fever. Crit Care 2016;20:200. [Crossref] [PubMed]
- Thomas-Rüddel DO, Hoffmann P, Schwarzkopf D, et al. Fever and hypothermia represent two populations of sepsis patients and are associated with outside temperature. Crit Care 2021;25:368. [Crossref] [PubMed]
- Shang Y, Madduma Hewage S, Wijerathne CUB, et al. Kidney Ischemia-Reperfusion Elicits Acute Liver Injury and Inflammatory Response. Front Med (Lausanne) 2020;7:201. [Crossref] [PubMed]
- Lane K, Dixon JJ, MacPhee IA, et al. Renohepatic crosstalk: does acute kidney injury cause liver dysfunction? Nephrol Dial Transplant 2013;28:1634-47. [Crossref] [PubMed]
- Jäger B, Drolz A, Michl B, et al. Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology 2012;56:2297-304. [Crossref] [PubMed]
- Bazick HS, Chang D, Mahadevappa K, et al. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med 2011;39:1913-21. [Crossref] [PubMed]
- Hu ZD, Lippi G, Montagnana M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: A narrative review. Clin Biochem 2020;77:1-6. [Crossref] [PubMed]
(English Language Editor: A. Kassem)