
High expression of the ANXA3 gene promotes immune infiltration and improves tumor prognosis in ovarian serous carcinoma using bioinformatics analyses
Introduction
Ovarian serous carcinoma (OV) is a serious, aggressive, and deadly gynecological carcinoma associated with a high recurrence rate, metastatic ability, and mortality and is resistant to conventional therapy (1). No effective method is available for early screening and diagnosis of OV. About 70% of patients are diagnosed in the advanced stage, and the 5-year survival rate is <25% for patients with stage III or IV OV (2). Although immunotherapy has recently been indicated for personalized treatment, the prognosis of advanced OV is still poor. Immune cells (3), mesenchymal cells (4), endothelial cells (5), inflammatory mediators (6), and extracellular matrix molecules contribute to the tumor microenvironment and dynamically regulate the immune response process. However, identifying immune-related genes that mediate OV prognosis may reveal new targets for OV immunotherapy. To improve the prognosis of OV, various predictive immune related biomarkers have been identified for better personalized treatments like Mesothelin (7), SLFN11 (8), B7-H4 and IDO1 (9) and so on. The new biomarkers need further verification due to some limitations like species difference, small sample size and other reasons. Bioinformatics research can often draw more accurate results based on large sample data in OV sample chip detect. Recent studies have shown that annexin A3 (ANXA3) gene expression is related to tumor aggressiveness and drug resistance (10). Few studies have reported on OV characteristics and therapeutic outcomes, and the role of ANXA3 in prognosis and immune infiltration in OV remains unclear. ANXA3 is a member of the annexin family of proteins and has 36-kDa and 33-kDa isoforms and closely related calcium (Ca2+)- and lipid-binding proteins. These are found at various intra- and extracellular locations, including human cumulus oophorus cells (11), and interact with a broad range of membrane lipids and proteins. Upregulation of ANXA3 expression in tumor tissues has been found to be closely associated with cell proliferation, migration, and apoptosis (12) via the phosphatidylinositol-3 kinase/protein kinase B (13), nuclear factor-κB (14), c-Jun (15), extracellular signal-regulated kinase, and hypoxia-inducible factor-1 signaling (16) pathways. These findings indicate that ANXA3 may serve as a novel diagnostic and prognostic biomarker for early tumor detection and population risk screening (17). There is now an abundance of published medical data available for multifactor regression modeling and updated prediction analysis, allowing progress with high validation in medical research (18). Consequently, this study developed an ANXA3 gene-based signature index to identify specific immune-related prognostic biomarkers to help find better predictive and therapeutic targets to improve the prognosis of OV patients. We present the following article in accordance with the TRIPOD reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3726/rc).
Methods
Data acquisition and processing
Patient datasets, with gene expression profiles and paired clinical information, were downloaded from The Cancer Genome Atlas (https://portal.gdc.cancer.gov) to verify the expression and prognostic impact of ANXA3 in OV based on the GPL570 platform tested by Affymetrix Human Genome U133 Plus 2.0 Array. Totally, the gene expression profile of 379 patients confirmed by histopathological examination with clinicopathological information (189 with low ANXA3 expression and 190 with high ANXA3 expression) were employed in the research (Table 1). All the patients had been administered at least one treatment of chemotherapy, radiotherapy or hormone therapy except surgery. Robust multi-array average method (19) was used with default options (with background correction, quantile normalization, and log transformation) to normalize raw data from batches by R/Bioconductor’s affy package. Using Xiantao Academic online platform (https://www.xiantao.love) to perform the bioinformatics and multivariate analysis to determine the role of ANXA3 on OV. This online platform comprises multiple modules, including basic mapping, difference analysis, functional clustering, interactive network, clinical significance, and other analysis modules. Bioinformatics analyses include gene expression difference, immune infiltrating lymphocyte subtype cell expression, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment and protein-protein interactions (PPIs) and were performed according to the self-contained software system. Indicators for prognostic evaluation include screening predictors, Kaplan-Meier survival curve and survival nomogram. The correlation of ANXA3 RNA sequencing with the clinicopathological information on the prognosis of OV was evaluated by Origin 9.1 (OriginLab Corporation, USA), a standard scientific data analysis and graphing software, to obtain a visible survival curve. The “interactive network” module of Xiantao Academic was used to further determine the relationships between ANXA3 expression and 23 tumor-infiltrating immune cells, including T helper cells, natural killer (NK) cells, Th1 and Th2 cells, macrophages, mast cells, T gamma delta (Tgd) cells, CD8+ T cells, plasmacytoid dendritic cells (pDCs), NK CD56 bright cells, NK CD56 dim cells, B cells, immature dendritic cells (iDCs), regulatory T cells (Treg), activated dendritic cells (aDCs), dendritic cells, T central memory (Tcm) cells, T effector memory (Tem) cells, T cells, cytotoxic cells, neutrophils, eosinophils, T follicular helper (TFH) cells, and Th17 cells. Three ovarian tumor datasets containing 1,691 samples were downloaded from the Gene Expression Omnibus repository to obtain the network of interacting proteins. The interactome consisted of the gene regulatory pathways to determine cellular behavior. PPIs among ANXA3 relevant proteins were established using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database. The signaling pathways of ANXA3 interactions were obtained by GO/KEGG enrichment analyses. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Table 1
| Characteristic | Low level of ANXA3 | High level of ANXA3 | P |
|---|---|---|---|
| Patients (n) | 189 | 190 | – |
| FIGO stage, n (%) | 0.330 | ||
| Stage I | 0 (0.0) | 1 (0.3) | |
| Stage II | 8 (2.1) | 15 (4.0) | |
| Stage III | 149 (39.6) | 146 (38.8) | |
| Stage IV | 30 (8.0) | 27 (7.2) | |
| Primary therapy outcome, n (%) | 0.898 | ||
| Progressive disease | 14 (4.5) | 13 (4.2) | |
| Stable disease | 11 (3.6) | 11 (3.6) | |
| Partial remission | 23 (7.5) | 20 (6.5) | |
| Complete response | 103 (33.4) | 113 (36.7) | |
| Race, n (%) | 0.401 | ||
| Asian | 6 (1.6) | 6 (1.6) | |
| Black or African American | 9 (2.5) | 16 (4.4) | |
| White | 164 (43.3) | 164 (43.3) | |
| Age (years), median (IQR) | 59.0 (51.0–69.0) | 58.5 (50.25–67.0) | 0.426 |
ANXA3, annexin A3; FIGO, International Federation of Gynecology and Obstetrics; IQR, interquartile range.
Statistical analysis
Data were expressed as the median (interquartile range) or means ± standard deviation (SD) for continuous variables and the rate/constituent ratio for frequency distribution and categorical variables using Origin 9.1 software for statistical analysis. After multiple logistic regression analysis, the Kaplan-Meier plotter was used for the survival data analysis. Correlations between clinical characteristics and ANXA3 expression were calculated using multiple regression analysis. The Cox logistic regression analysis was used to identify the overall survival-related clinical characteristics of patients with OV. The correlation analysis between ANXA3 gene expression and immune cell infiltration was assessed using Spearman’s R. A P value <0.05 indicated a statistically significant difference.
Results
Survival outcomes and multivariate analysis
The results indicated that the expression of ANXA3 was abnormal in patients with OV. The upregulated ANXA3 expression level significantly correlated with a good overall survival [Figure 1A, hazard ratio (HR) =0.69, χ2=6.48, P=0.011] and a markedly high ANXA3 expression was present in OV tissues compared with normal tissues [Figure 1B, 1.83 (1.387–2.21) vs. 5.138 (4.454–5.849), t=589.5, P<0.001]. As shown in Figure 1C, unpaired-sample data studies show that the expression level of ANXA3 significantly increased in patients with lymphatic invasion compared with those with non-lymphatic invasion (4.947±0.983 vs. 4.588±0.983, t=2.08, P=0.039). Due to the lack of paired OV data and the possibility of decreased expression of ANXA3 in any other tumors, the differences in ANXA3 RNAseq data were detected using the Wilcoxon signed-rank test (Figure 1D). Compared with matched healthy controls, the ANXA3 expression in tumor tissues decreased significantly in breast cancer (BRCA, n=112, P<0.001), kidney chromophobe (KICH, n=23, P<0.001), kidney renal clear cell carcinoma (KIRC, n=72, P<0.001), liver hepatocellular carcinoma (LIHC, n=50, P<0.001), lung adenocarcinoma (LUAD, n=57, P<0.001), lung squamous cell carcinoma (LUSC, n=49, P<0.001), and thyroid carcinoma (n=58, P<0.001) and markedly increased in other tumor tissues, including cholangiocarcinoma (CHOL, n=9, P=0.004), colon adenocarcinoma (COAD, n=41, P<0.001), prostate adenocarcinoma (PRAD, n=52, P=0.007), and rectum adenocarcinoma (READ, n=9, P=0.004). In addition, multiple regression analysis (Figure 2A) was performed to detect the association between ANXA3 expression levels and OV clinical variables. The results indicated that the Federation International of Gynecology and Obstetrics (FIGO) stage (HR =2.495, P=0.037), primary therapy outcome including stable disease (SD) and complete response (CR) (HRSD =0.441 and HRCR =0.152, all P<0.05), age (HR =1.355, P=0.021), and residual tumor (HR =2.313, P<0.001) could be used as independent prognostic factors, whereas the ANXA3 expression level could not independently predict OV prognosis (HR =0.901, P=0.430). Using the nomogram prediction model, multiple prediction indexes were integrated (Figure 2A), and then the segmentation levels with scales were converted into weighted standard scores. In the end, the total survival period was predicted to be 2.25 years from initial OV diagnosis, and the overall 1-year survival rate in all patients was calculated to be 80%, with a total weighted score of 173 points (Figure 2B).
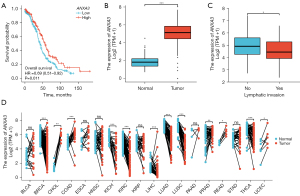

Association between ANXA3 gene expression and clinicopathological variables
The ANXA3 gene could not act as an independent predictor of OV, suggesting that ANXA3 might interact with other clinical characteristics and be beneficial to the prognosis of OV. Hence, this study investigated the associations between ANXA3 levels with the FIGO stage, primary therapy outcome, race, age, histologic grade, anatomic neoplasm subdivision, venous or lymphatic invasion, residual tumor, and tumor status. As shown in Table 2, a low expression of ANXA3 correlated significantly with lymphatic invasion (OR =0.448, P=0.025).
Table 2
| Characteristics | Total (n) | Odds ratio (95% CI) | P |
|---|---|---|---|
| FIGO stage (stages III and IV vs. stages I and II) | 376 | 0.483 (0.192–1.128) | 0.103 |
| Primary therapy outcome (CR vs. PD, SD, and PR) | 308 | 1.197 (0.734–1.954) | 0.471 |
| Race (White vs. Asian, Black, or African American) | 365 | 0.682 (0.336–1.351) | 0.277 |
| Age (>60 vs. ≤60 years) | 377 | 0.890 (0.593–1.335) | 0.574 |
| Histologic grade (G3 and G4 vs. G1 and G2) | 369 | 0.579 (0.303–1.079) | 0.089 |
| Anatomic neoplasm subdivision (bilateral vs. unilateral) | 357 | 0.778 (0.489–1.231) | 0.285 |
| Venous invasion (yes vs. no) | 105 | 0.467 (0.207–1.031) | 0.062 |
| Lymphatic invasion (yes vs. no) | 149 | 0.448 (0.219–0.897) | 0.025 |
| Residual tumor (RD vs. NRD) | 335 | 0.914 (0.533–1.563) | 0.743 |
| Tumor status (with tumor vs. tumor free) | 337 | 1.098 (0.651–1.853) | 0.726 |
ANXA3, annexin A3; FIGO, International Federation of Gynecology and Obstetrics; PD, progressive disease; SD, stable disease; PR, partial remission; CR, complete response; RD, residual disease; NRD, no residual disease.
Relationship between ANXA3 gene expression and tumor-infiltrating immune cells
This study indicated that ANXA3 expression correlated positively with T helper cells, NK cells, Th1 cells, and Th2 cells and correlated negatively with macrophages, mast cells, Tgd cells, CD8 T cells, pDC, NK CD56 bright cells, NK CD56 dim cells, B cells, iDCs, Treg, aDCs, DCs, Tcm cells, Tem cells, T cells, cytotoxic cells, neutrophils, eosinophils, and TFH cells. The Pearson’s correlation analysis showed that the differences achieved statistical significance only in Th17 cells (r=–0.169, P<0.001), TFH cells (r=–0.129, P=0.012), and Tem cells (r=–0.104, P=0.043) (Figure 3A). The upregulation of ANXA3 promoted the activity of NK cells and inhibited the activity of other lymphocytes, especially three lymphocyte subtypes: Th17 cells, TFH cells, and Tem cells. Additionally, the difference in ANXA3 low and high expression in specific lymphocyte subsets (Figure 3) was analyzed using the Mann-Whitney U test (Table 3) in OV tissues to determine whether ANXA3 expression in different lymphocyte subtypes in the specific location mainly increased or decreased. The results revealed marked low expression compared with high expression in T cells (0.324±0.113 vs. 0.285±0.125, P=0.003) and B cells (0.194±0.067 vs. 0.175±0.073, P=0.022). The same statistical results showed that low expression was more significant than high expression in Th17 cells (0.18±0.093 vs. 0.153±0.099, P=0.009) and TFH cells (0.322±0.037 vs. 0.310±0.039, P=0.010); no significant differences were found in NK cells (P=0.146), macrophages (P=0.114), or Tem cells (P=0.076).

Table 3
| Lymphocyte subtypes | High (n=190) | Low (n=189) | Z | P |
|---|---|---|---|---|
| T cells | 0.285±0.125 | 0.324±0.113 | 14,838 | 0.003 |
| B cells | 0.175±0.073 | 0.194±0.067 | 15,513 | 0.022 |
| Macrophages | 0.524±0.069 | 0.535±0.06 | 16,267 | 0.114 |
| NK cells | 0.527±0.026 | 0.524±0.025 | 19,507 | 0.146 |
| Th17 cells | 0.153±0.099 | 0.18±0.093 | 15,149 | 0.009 |
| TFH cells | 0.31±0.039 | 0.322±0.037 | 15,222 | 0.010 |
| Tem cells | 0.406±0.039 | 0.412±0.038 | 16,065 | 0.076 |
Means and standard deviation of ANXA3 mRNA expression values in different groups. ANXA3, annexin A3; NK, natural killer; TFH, T follicular helper; Tem, T effector memory.
ANXA3-related protein interaction network and GO/KEGG signaling pathway enrichment analysis
The PPI revealed the four most essential ANXA3-associated molecular markers of lymphatics: CD63, CD81, CD82, and CD9, and six additional functional genes: CASP3, TSG101, STX4, STXBP2, SNAP23, and ANXA11 based on the differential expression (Figure 4A). The GO/KEGG analysis of ANXA3-related genes revealed 10 significantly enriched pathways in OV. Most of the pathways were related to immune functions and response to OV (Figure 4B). Table 4 summarizes the most important sets of gene categories and their participating biological processes in OV. Among these, most of the genes belonged to the intracellular signaling cascade pathways directly participating in extracellular vesicle (EV) secretion (SNAP23, STX4, STXBP2, CD9, CD63, and CD81) or indirectly acting on the decisive promoting ion Ca2+ affecting the secretion of EVs through phospholipase A2/Ca2+ and p53-mediated apoptosis signaling pathway (P53 and CASP3), regulating the downstream cascade, except one molecule related to the pathway of the classical lymphocyte infiltration process represented by CD82.
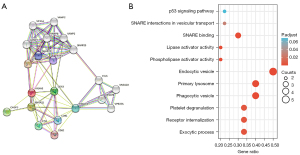
Table 4
| Ontology | ID | Description | P value | Gene ID |
|---|---|---|---|---|
| BP | GO:0140029 | Exocytic process | 6.25e−06 | STX4/STXBP2/SNAP23 |
| BP | GO:0031623 | Receptor internalization | 1.67e−05 | CD9/CD63/CD81 |
| BP | GO:0002576 | Platelet degranulation | 2.57e−05 | CD9/CD63/STXBP2 |
| CC | GO:0030139 | Endocytic vesicle | 1.96e−07 | ANXA11/CD9/STX4/STXBP2/SNAP23 |
| CC | GO:0045335 | Phagocytic vesicle | 3.91e−07 | ANXA11/STX4/STXBP2/SNAP23 |
| CC | GO:0005766 | Primary lysosome | 7.44e−07 | ANXA11/CD63/STXBP2/SNAP23 |
| MF | GO:0016004 | Phospholipase activator activity | 1.89e−05 | CASP3/STX4 |
| MF | GO:0060229 | Lipase activator activity | 2.61e−05 | CASP3/STX4 |
| MF | GO:0000149 | SNARE binding | 2.72e−05 | STX4/STXBP2/SNAP23 |
| KEGG | hsa04130 | SNARE interactions in vesicular transport | 5.73e−04 | STX4/SNAP23 |
| KEGG | hsa04115 | p53 signaling pathway | 0.003 | CASP3/CD82 |
BP, biological process; CC, cellular component; MF, molecular function; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Discussion
OV is one of the most common epithelial malignant tumors in the female reproductive system, accounting for about 30% of all ovarian carcinomas (20). Surgery, chemotherapy, radiotherapy, targeted therapy, biotherapy, and other treatment approaches are used after clinical diagnosis. However, it is difficult to achieve a complete cure, and more than 50% of patients die within 6 years (21). Considering the strong invasive and metastatic characteristics of OV with poor prognosis, more potent therapeutic and prognostic predictors related to the genetic, endocrine, gynecological disease, fertility, lifestyle, and other risk factors need to be explored.
Inflammation is closely related to the occurrence and development of OV (22). To date, ANXA3 has been found to not only maintain genomic integrity and facilitate cell proliferation and differentiation but also contributes to tumor initiation and growth. We synthesized the relevant literature and found that that ANXA3 expression was dysregulated in a large number of tumor populations, such as bladder urothelial carcinoma (23), BRCA (17), CHOL (16), COAD (24), esophageal carcinoma (25), head and neck squamous cell carcinoma (26), kidney chromophobe (27), kidney renal papillary cell carcinoma (28), hepatocellular carcinoma (29), LUAD (30), LUSC (31), pancreatic adenocarcinoma (32), PRAD (33), READ (34), stomach adenocarcinoma (25), thyroid carcinoma (35), and uterine corpus endometrial carcinoma (13). In this study, an increased ANXA3 expression was associated with a favorable prognosis, suggesting that ANXA3 might play a pivotal role in decreasing cancer risk in patients with OV. Additionally, a multiple logistic regression analysis was used to determine the predictors of tumor prognosis, including ANXA3. The results revealed that FIGO stage, primary therapy outcome, age, and residual tumor factors significantly predicted overall survival in OV, but the ANXA3 expression level did not independently predict prognosis (P>0.05). The total life span when the factors above worked together to predict overall survival was calculated to be 2.25 years after initial diagnosis, and the overall 1-year survival rate was about 80% using a nomogram evaluation. As ANXA3 was not an independent predictor, the correlation between ANXA3 and other independent predictors was further analyzed. The results showed that high ANXA3 expression significantly correlated with lymphatic invasion, implying that ANXA3 acted on local lymph infiltration and distant lymph node metastasis, thus influencing the prognosis of patients.
The process of lymphatic infiltration in tumors involves different lymphocyte subtypes and is associated with not only tumor diffusion and metastasis but also the immune response of the body to tumor cells. To date, many targeted drugs and immune cell therapies have been developed for clinical immunotherapy based on the research progress in lymphatic infiltration. This study verified the correlation between lymphocyte subsets and ANXA3 gene expression to confirm the potential of different immune cells during lymphatic infiltration in OV. The findings revealed that ANXA3 expression levels correlated positively with the number of T helper cells, NK cells, Th1 cells, and Th2 cells but correlated negatively with macrophages, mast cells, Tgd cells, CD8 T cells, pDCs, NK CD56 bright cells, NK CD56 dim cells, B cells, iDCs, Treg, aDCs, DCs, Tcm cells, Tem cells, T cells, cytotoxic cells, neutrophils, eosinophils, TFH cells, and Th17 cells. Further, an increased ANXA3 expression promoted the effects of NK cells and cytotoxic T lymphocyte functioning, which was beneficial to clinical immunotherapy. However, this promoting effect did not reach statistical significance. The significant correlation between ANXA3 and lymphocyte subtypes appeared only in TFH cells, Th17 cells, and Tem lymphocyte subtypes, revealing that TFH- and Th17-cell-mediated immunity and pathology may highlight potential targets for OV therapies in the future.
TFH cells promote the survival, proliferation, and differentiation of B cells into immunoglobulin-producing cells and generate protective antibodies against various infections (36) and harmful stimulators (37). They also play a key role in inducing T-cell effectors to recruit activated cytotoxic NK and CD8+ T cells in tumors (38). The dysregulation of TFH cell responses in tumors is thought to be related to the recognition of immune checkpoints, such as programmed death protein-1 (PD-1), B cell lymphoma 6, C-C chemokine receptor 7, and C-X-C chemokine receptor type 5 (39). Recent studies have shown that other Th subsets, including Th1, Th2, Th9, and Treg, may become TFH cells under specific pathological conditions (40) and participate in the dynamic balance between Th1 and Th2 cells, mediating immunotoxicity and immunosuppression. Th1, Th9, and TFH cells stimulate the antitumor immune response, while Th2 and Treg cells routinely induce immunosuppressive protumorigenic responses. Th17 cells also contribute to the delicate balance between Th1 and Th2 anti- versus pro-tumorigenic networks and play a dual role in impairing immune functions by targeting granzyme B production, a dominant marker for cytolytic CD4+ activity (41). Currently, the exact proportion and change in TFH and Th17 cell expression involved in lymphatic infiltration in ovarian tumor tissues, nontumor tissues, and invaded lymph nodes is unclear. This information is of great value for immunotherapy in OV and should be clarified in further research. Given the double-edged effects of ANXA3 in lymphatic infiltration when moderately activated on the swelling of invaded tumor cells, once over activated, the lymph infiltration will produce a strong inflammatory reaction damaging normal tissue DNA (42) and its role in inducing the risk of tumor cell proliferation through a complex network of chemokines (43). This study also compared ANXA3 expression in different lymphocyte subtypes. The results revealed that low ANXA3 expression in TFH and Th17 cells was more predominant than high expression. This finding indicated that increased ANXA3 expression promoted the downregulation of TFH and Th17 cells, improved lymphocyte immune infiltration, and enhanced the prognosis of patients with OV in precise patterns. This feature was consistent with the fact that a moderate immune response had a protective effect while an excessive immune response caused more damage to patients.
PPI maps help researchers determine the interacting protein genes and gain an understanding of specific signaling pathways. Using the STRING database, 10 ANXA3-related proteins were identified in this study: CD63, CD81, CD82, CD9, CASP3, TSG101, STX4, STXBP2, SNAP23, and ANXA11. The GO/KEGG enrichment analysis revealed three dominant specific pathways for the regulation of EV interactions with ANXA3: (I) the Ca2+ release-promoting signaling pathway represented by phospholipase A2; (II) the life cycle signaling involved in EV assembly, recognition, transportation, and reuptake processes (44); and (III) p53-mediated apoptosis. EVs are categorized into three forms: microvesicles, exosomes, and apoptotic bodies (45). In recent years, the immunobiological and functional research concerning EVs has made significant progress. For example, as the main component of oncosomes, tetraspanins (CD9 and CD81) function to cleave large cytoplasmic extensions from the cell body for constitutive EV secretion (46). CD63+ lysosome-related effector vesicles contain protease. Perforin may be used by cytotoxic T lymphocytes and NK cells to fight against BRCA and also transports diverse immunomodulatory proteins, including major histocompatibility complex (MHC) classes I and II, costimulatory and adhesion molecules, enzymes, or cytokines (47). EVs transport programmed death protein-ligand 1 (PD-L1), which interacts with PD-1 and reduces CD8+ T-cell proliferation and promotes T-cell apoptosis, which in turn reduces the immune response and leads to large-scale tumor growth. PD-L1 also blocks Ca2+/soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex induction and regulates exosome secretion (48). EVs carrying the CD95 ligand or tumor necrosis factor-related apoptosis-inducing ligand can induce the apoptosis of activated T cells via p53 to expose PD-L1, thereby inhibiting T-cell-mediated immunity against cancers (49). Given their convenient delivery via blood circulation and ability to cross biological barriers (50), exosomes released from chimeric antigen receptor T lymphocytes exhibit excellent potential as direct attackers in immunotherapy. These findings suggest that the interaction between ANXA3 and EVs may affect the efficacy of immunotherapy and the prognosis of OV, although this requires further validation in subsequent studies.
This paper has some limitations. Firstly, the study aimed to identify important predictors using a multiple regression model in a multivariable analysis. However, the methodology and predictive accuracy of the model requires improvement. Secondly, only one standard data set was retrieved in this study and the limited number of cases were included also needs to expand the sample size to further strengthen the intensity in this study. In summary, increased ANXA3 expression correlates with a favorable prognosis in OV, which may be achieved by promoting the infiltration of TFH and Th17 lymphocytes or by acting on EVs.
Conclusions
ANXA3 is responsible for the complexity of lymphatic infiltration related to OV outcomes. It induces a stronger T-cell-mediated immunity against tumor cells, implying that it can be used as an immunotherapy target.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3726/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3726/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiang MM, Zhao F, Lou TT. Assessment of Significant Pathway Signaling and Prognostic Value of GNG11 in Ovarian Serous Cystadenocarcinoma. Int J Gen Med 2021;14:2329-41. [Crossref] [PubMed]
- Wang H, Ye F, Zhou C, et al. High expression of ENPP1 in high-grade serous ovarian carcinoma predicts poor prognosis and as a molecular therapy target. PLoS One 2021;16:e0245733. [Crossref] [PubMed]
- Communal L, Roy N, Cahuzac M, et al. A Keratin 7 and E-Cadherin Signature Is Highly Predictive of Tubo-Ovarian High-Grade Serous Carcinoma Prognosis. Int J Mol Sci 2021;22:5325. [Crossref] [PubMed]
- Otsuka I. Mechanisms of High-Grade Serous Carcinogenesis in the Fallopian Tube and Ovary: Current Hypotheses, Etiologic Factors, and Molecular Alterations. Int J Mol Sci 2021;22:4409. [Crossref] [PubMed]
- Escalona RM, Kannourakis G, Findlay JK, et al. Expression of TIMPs and MMPs in Ovarian Tumors, Ascites, Ascites-Derived Cells, and Cancer Cell Lines: Characteristic Modulatory Response Before and After Chemotherapy Treatment. Front Oncol 2021;11:796588. [Crossref] [PubMed]
- Pietilä EA, Gonzalez-Molina J, Moyano-Galceran L, et al. Co-evolution of matrisome and adaptive adhesion dynamics drives ovarian cancer chemoresistance. Nat Commun 2021;12:3904. [Crossref] [PubMed]
- Giordano G, Ferioli E, Tafuni A. The Role of Mesothelin Expression in Serous Ovarian Carcinoma: Impacts on Diagnosis, Prognosis, and Therapeutic Targets. Cancers (Basel) 2022;14:2283. [Crossref] [PubMed]
- Winkler C, King M, Berthe J, et al. SLFN11 captures cancer-immunity interactions associated with platinum sensitivity in high-grade serous ovarian cancer. JCI Insight 2021;6:146098. [Crossref] [PubMed]
- Niu N, Shen W, Zhong Y, et al. Expression of B7-H4 and IDO1 is associated with drug resistance and poor prognosis in high-grade serous ovarian carcinomas. Hum Pathol 2021;113:20-7. [Crossref] [PubMed]
- Guo C, Li N, Dong C, et al. 33-kDa ANXA3 isoform contributes to hepatocarcinogenesis via modulating ERK, PI3K/Akt-HIF and intrinsic apoptosis pathways. J Adv Res 2021;30:85-102. [Crossref] [PubMed]
- Chermuła B, Hutchings G, Kranc W, et al. Expression Profile of New Gene Markers and Signaling Pathways Involved in Immunological Processes in Human Cumulus-Oophorus Cells. Genes (Basel) 2021;12:1369. [Crossref] [PubMed]
- Liu C, Li N, Liu G, et al. Annexin A3 and cancer. Oncol Lett 2021;22:834. [Crossref] [PubMed]
- Wu F, Yang J, Liu J, et al. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther 2021;6:218. [Crossref] [PubMed]
- Zhang Z, Li Z, Ma Z, et al. Annexin A3 as a Marker Protein for Microglia in the Central Nervous System of Rats. Neural Plast 2021;2021:5575090. [Crossref] [PubMed]
- Tam SY, Law HK. JNK in Tumor Microenvironment: Present Findings and Challenges in Clinical Translation. Cancers (Basel) 2021;13:2196. [Crossref] [PubMed]
- Wang F, Breslin S J P, Qiu W. Novel oncogenes and tumor suppressor genes in hepatocellular carcinoma. Liver Res 2021;5:195-203. [Crossref] [PubMed]
- Yang L, Lu P, Yang X, et al. Annexin A3, a Calcium-Dependent Phospholipid-Binding Protein: Implication in Cancer. Front Mol Biosci 2021;8:716415. [Crossref] [PubMed]
- Collins GS, Dhiman P, Andaur Navarro CL, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021;11:e048008. [Crossref] [PubMed]
- Albaradei S, Thafar M, Alsaedi A, et al. Machine learning and deep learning methods that use omics data for metastasis prediction. Comput Struct Biotechnol J 2021;19:5008-18. [Crossref] [PubMed]
- Zhang Y, Du T, Chen X. ANXA2P2: A Potential Immunological and Prognostic Signature in Ovarian Serous Cystadenocarcinoma via Pan-Carcinoma Synthesis. Front Oncol 2022;12:818977. [Crossref] [PubMed]
- Canals Hernaez D, Hughes MR, Dean P, et al. PODO447: a novel antibody to a tumor-restricted epitope on the cancer antigen podocalyxin. J Immunother Cancer 2020;8:e001128. [Crossref] [PubMed]
- Peres LC, Townsend MK, Birmann BM, et al. Circulating Biomarkers of Inflammation and Ovarian Cancer Risk in the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev 2021;30:710-8. [Crossref] [PubMed]
- Yao X, Qi X, Wang Y, et al. Identification and Validation of an Annexin-Related Prognostic Signature and Therapeutic Targets for Bladder Cancer: Integrative Analysis. Biology (Basel) 2022;11:259. [Crossref] [PubMed]
- Luparello C. Cadmium-Associated Molecular Signatures in Cancer Cell Models. Cancers (Basel) 2021;13:2823. [Crossref] [PubMed]
- Grewal T, Rentero C, Enrich C, et al. Annexin Animal Models-From Fundamental Principles to Translational Research. Int J Mol Sci 2021;22:3439. [Crossref] [PubMed]
- Chen N, He D, Cui J. A Neutrophil Extracellular Traps Signature Predicts the Clinical Outcomes and Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Front Mol Biosci 2022;9:833771. [Crossref] [PubMed]
- Zhang Y, Liu Q, Liu J, et al. Upregulated CD58 is associated with clinicopathological characteristics and poor prognosis of patients with pancreatic ductal adenocarcinoma. Cancer Cell Int 2021;21:327. [Crossref] [PubMed]
- Zhang C, Zhang W, Cui H, et al. Role of Hub Genes in the Occurrence and Development of Testicular Cancer Based on Bioinformatics. Int J Gen Med 2022;15:645-60. [Crossref] [PubMed]
- Romualdo GR, Leroy K, Costa CJS, et al. In Vivo and In Vitro Models of Hepatocellular Carcinoma: Current Strategies for Translational Modeling. Cancers (Basel) 2021;13:5583. [Crossref] [PubMed]
- Chen Q, Wang X, Hu J. Systematically integrative analysis identifies diagnostic and prognostic candidates and small-molecule drugs for lung adenocarcinoma. Transl Cancer Res 2021;10:3619-46. [Crossref] [PubMed]
- Chen C, Hou J, Yu S, et al. Role of cancer-associated fibroblasts in the resistance to antitumor therapy, and their potential therapeutic mechanisms in non-small cell lung cancer. Oncol Lett 2021;21:413. [Crossref] [PubMed]
- Hendley AM, Rao AA, Leonhardt L, et al. Single-cell transcriptome analysis defines heterogeneity of the murine pancreatic ductal tree. Elife 2021;10:67776. [Crossref] [PubMed]
- Ruan Y, Xu H, Ji X, et al. BLM interaction with EZH2 regulates MDM2 expression and is a poor prognostic biomarker for prostate cancer. Am J Cancer Res 2021;11:1347-68. [PubMed]
- De P, Aske J, Dey N. Cancer-Associated Fibroblast Functions as a Road-Block in Cancer Therapy. Cancers (Basel) 2021;13:5246. [Crossref] [PubMed]
- Baagar KA, Alowainati BI. Papillary Thyroid Cancer Affecting Multiple Family Members: A Case Report and Literature Review of Familial Nonmedullary Thyroid Cancer. Case Rep Endocrinol 2021;2021:3472000. [Crossref] [PubMed]
- Schultheiß C, Paschold L, Simnica D, et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity 2020;53:442-455.e4. [Crossref] [PubMed]
- Cirelli KM, Carnathan DG, Nogal B, et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019;177:1153-1171.e28. [Crossref] [PubMed]
- Bengsch B, Ohtani T, Herati RS, et al. Deep immune profiling by mass cytometry links human T and NK cell differentiation and cytotoxic molecule expression patterns. J Immunol Methods 2018;453:3-10. [Crossref] [PubMed]
- Onabajo OO, George J, Lewis MG, et al. Rhesus macaque lymph node PD-1(hi)CD4+ T cells express high levels of CXCR5 and IL-21 and display a CCR7(lo)ICOS+Bcl6+ T-follicular helper (Tfh) cell phenotype. PLoS One 2013;8:e59758. [Crossref] [PubMed]
- Wang W, Sung N, Gilman-Sachs A, et al. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front Immunol 2020;11:2025. [Crossref] [PubMed]
- Park S, Anderson NL, Canaria DA, et al. Granzyme-Producing CD4 T Cells in Cancer and Autoimmune Disease. Immunohorizons 2021;5:909-17. [Crossref] [PubMed]
- Shi F, Deng T, Mo J, et al. An Immune-Related Gene-Based Signature as Prognostic Tool in Ovarian Serous Cystadenocarcinoma. Int J Gen Med 2021;14:4095-104. [Crossref] [PubMed]
- Chen YF, Shao GC, Li J, et al. O-GlcNAcylation of Blimp-1 in Lymphocytes Inhibits Its Transcriptional Function and Is Associated with Migration and Invasion of Breast Cancer Cells. Mol Cancer Res 2022;20:650-60. [Crossref] [PubMed]
- Calvo V, Izquierdo M. Inducible Polarized Secretion of Exosomes in T and B Lymphocytes. Int J Mol Sci 2020;21:2631. [Crossref] [PubMed]
- Beck S, Hochreiter B, Schmid JA. Extracellular Vesicles Linking Inflammation, Cancer and Thrombotic Risks. Front Cell Dev Biol 2022;10:859863. [Crossref] [PubMed]
- Samuels M, Cilibrasi C, Papanastasopoulos P, et al. Extracellular Vesicles as Mediators of Therapy Resistance in the Breast Cancer Microenvironment. Biomolecules 2022;12:132. [Crossref] [PubMed]
- Lettau M, Janssen O. Intra- and Extracellular Effector Vesicles From Human T And NK Cells: Same-Same, but Different? Front Immunol 2021;12:804895. [Crossref] [PubMed]
- Liu J, Peng X, Yang S, et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: biological function and potential therapy strategies. Cell Commun Signal 2022;20:14. [Crossref] [PubMed]
- Sanaei M, Kavoosi F. Effects of trichostatin A on the intrinsic and extrinsic apoptotic pathway, cell viability, and apoptosis induction in hepatocellular carcinoma cell lines. Gastroenterol Hepatol Bed Bench 2021;14:323-33. [PubMed]
- Record M, Attia M, Carayon K, et al. Targeting the liver X receptor with dendrogenin A differentiates tumour cells to secrete immunogenic exosome-enriched vesicles. J Extracell Vesicles 2022;11:e12211. [Crossref] [PubMed]
(English Language Editor: D. Fitzgerald)


