
Anlotinib combined with chemotherapy and immunotherapy for advanced pulmonary sarcomatoid cancer: a case report and literature review
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare, highly malignant type of pulmonary cancer with a poor prognosis which accounts for approximately 0.1–0.4% of all lung cancer cases (1). This rare subtype of poorly differentiated non-small cell lung cancer (NSCLC) contains sarcoma-like elements including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and pulmonary blastoma (2). The principles of the treatment for sarcomatoid lung cancer are the same as those for other NSCLCs. Surgery is the first choice of treatment when clinically appropriate, but the role of radiation is controversial and associated with poor efficacy in some patients. This is mainly because radiotherapy is only used in patients with stage I–III non-advanced PSC. Due to the low radiosensitivity of PSC, the effectiveness of radiotherapy in the treatment of PSC patients is limited. First-line chemotherapy most commonly includes platinum-based doublets with either pemetrexed, paclitaxel, docetaxel, gemcitabine or vinorelbine. The overall survival (OS) of patients treated with platinum-based doublet chemotherapy is higher than that of those treated with single agent chemotherapy without platinum. However, chemotherapy generally has limited efficacy in the treatment of PSC. In a retrospective study of 97 patients, patients with advanced PSC treated with chemotherapy had a median PFS of only 2months and a median OS of 6.3 months (3). It can be seen that traditional chemotherapy has limited benefit for advanced PSC.
With advances in research on tumor molecular mechanisms, the molecular characteristics of PSC suggest that molecular targeted therapy may be a potential therapeutic strategy for PSC, a rare with strong inter- and intratumor heterogeneity (4). Studies have shown that epithelial-mesenchymal transition plays an important role in the carcinogenesis of PSC. A pan-cancer analysis revealed a high tumor mutational burden in PSC (5). In PSC, mesenchymal-epithelial transition (MET) exon-14 skipping account for approximately 9.5–22% of the number of patients with PSC (6,7).
There are several reports that patients with MET gene mutated advanced NSCLC treated with MET inhibitors such as crizotinib, capmatinib and tepotinib, have shown a good response (8). Antiangiogenic inhibitors, such as anlotinib which is a novel multi-target tyrosine kinase inhibitor (TKI) that targets the vascular endothelial growth factor receptor (VEGFR), play an important role in the palliative treatment of advanced solid tumors including NSCLC. A study has shown that vascular invasion is a major factor in the poor prognosis of PSC patients (9). Recently, some studies have reported a high incidence of programmed death-ligand 1 (PD-L1) expression in PSC tumors and the PD-L1 levels of PSC are often higher than those of other NSCLCs (10,11). According to a pooled analysis by Babacan et al., PD-L1 expression is associated with increased tumor response and improved progression free survival (PFS) to immunotherapy with immune checkpoint inhibitors (ICIs), even when the PD-L1 level is <1%, immunotherapy can still be effective (12). Thus, immunotherapy has become a breakthrough treatment for PSC. Several case reports have been reported before and after using ICIs, such as toripalimab, camrelizumab, nivolumab, pembrolizumab. According to a retrospective study of 37 patients with PSC, the objective response rate (ORR) was 40.5% and the disease control rate (DCR) was 64.8%, regardless of PD-L1 status. The median OS was 12.7 months, which is significantly longer than chemotherapy (13). Although there have been anecdotal reports on the use of ICIs to treat PSC, data from prospective clinical studies of ICIs in patients with PSC are still lacking (14). Further, little is known about which ICI to use and the optimal treatment duration. Thus, while the emergence of targeted therapy and immunotherapy has extended the survival of PSC patients, the current evidence about these treatment modalities for PSC remains limited. In the present study, we report the dramatic response of a PSC patients with comprehensive treatments, include chemotherapy, anlotinib, immunotherapy. This rare case may provide inspiration for individualized treatment of patients with advanced PSC. In particular, the efficacy and safety of long-term maintenance therapy with immunological and antiangiogenic therapies can be demonstrated. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4312/rc).
Case presentation
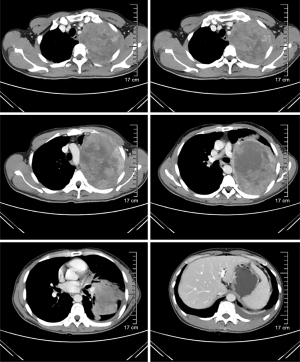
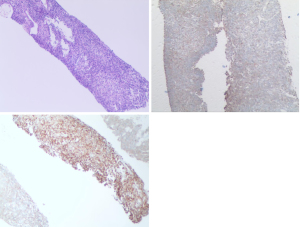
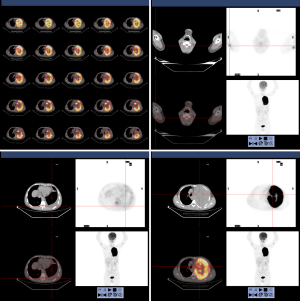
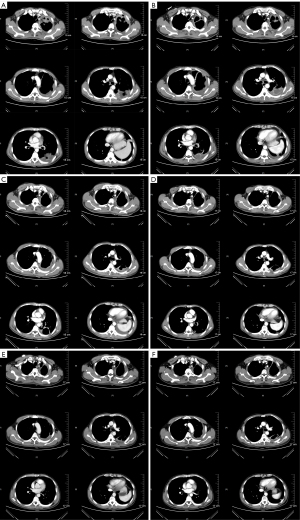
In April 2020, a 42-year-old, non-smoking male patient, who was previously healthy without a history of other chronic, infectious or genetic diseases, was admitted to our department (Department of Radiation Oncology, The Affiliated Hospital of Soochow University) for left-side back pain radiating to the ipsilateral side of his chest and armpit, accompanied by left upper limb numbness. A contrast-enhanced computed tomography (CT) scan of the chest showed a large mass (159 mm × 112 mm) in the lower lobe of the left lung, which was considered a possible malignancy with vascular invasion (Figure 1). We performed an image-guided percutaneous needle core biopsy of the lung tumor on May 18, 2020. Histopathologic examination together with immunohistochemical staining results (Figure 2), suggest a diagnosis of sarcomatoid carcinoma but the possibility of a sarcoma with epithelioid differentiation could not be ruled out. The immunohistochemistry results were as follows: cancer cell cytokeratin (CK) (+), Vimentin (+), thyroid transcription factor-1 (TTF-1) (focal +), transducin-like enhancer of split 1 (TLE-1) (focal +), PD-L1 (22C3) (+70%), Ki-67 (+60%), leukocyte common antigen (LCA) (−), S100 (−), CK7 (−), napsin A (−), CK5/6 (−), P40 (−), anaplastic lymphoma kinase (ALK) (D5F3) (−), synapsin (Syn) (−), P63 (−), B-cell lymphoma-2 (Bcl-2) (−), and cluster of differentiation 34 (CD34) (−). Fluorodeoxyglucose positron emission tomography (PET)-CT performed on June 11, 2020 and showed a large tumor in the left lung with increased glucose metabolism. The right axillary lymph nodes and subcutaneous nodules of both upper arms were hypermetabolic and metastasis to these sites was considered. PET-CT also showed a large left pleural effusion compressing the heart and mediastinum which were shifted to the right (Figure 3).
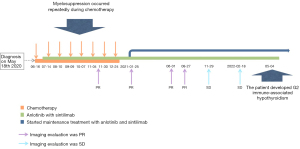
According to the 8th edition of the American Joint Committee on Cancer (AJCC)/Union International Contre le Cancer (UICC) TNM staging system for lung cancer, the patient was diagnosed with PSC of the left lung (T4N2M1c, stage IVB) with metastasis to the right axillary lymph nodes, subcutaneous tissues of both upper arms, and the left pleura. The patient’s Eastern Cooperative Oncology Group performance status score was 1. Thus, the first cycle of chemotherapy comprising abraxane [300 mg on day 1 (D1)] and cisplatin (50 mg D1–D2) on June 16, 2020. Unfortunately, the disease progressed, and the patient developed chest tightness and shortness of breath. An emergency chest CT showed that the left lung mass (166 mm × 117 mm) was larger than before (Figure 4). The patient’s condition continued to deteriorate with his oxygen saturation by pulse oximetry dropping to 76% in some instances despite supplemental oxygen therapy. The patient’s dyspnea was relieved with drainage of the large left-sided pleural effusion under ultrasound guidance. A total of 4,620 mL of dark red bloody fluid was drained. We then decided to begin targeted therapy combined with immunotherapy and chemotherapy. On July 11, 2020 the patient began taking anlotinib [12 mg peros (po) quarter in die (qd) days 1–14, 3weeks using a (q3w)]. On July 14, the patient was commenced on immunotherapy combined with chemotherapy consisting of sintilizumab (200 mg D1), abraxane (150 mg D1, D8), and cisplatin (25 mg D1–D2, D8–D9). During the course of treatment, the patient’s symptoms of chest tightness and dyspnea gradually improved. On August 10, September 9, October 7, November 4, November 30, and December 24, 2020 the patient received a combined treatment of anlotinib (12 mg qd for 14 days), sintilizumab (200 mg D1), abraxane (150 mg D1, D8), and cisplatin (25 mg D1–D2, D8–D9).
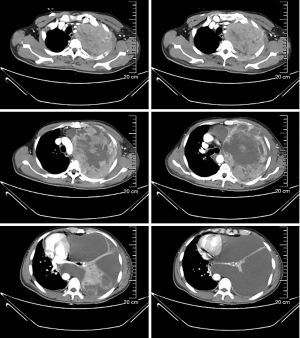
During the course of the disease, especially after chemotherapy, the patient had a poor appetite and fatigue, which became more prominent with subsequent treatment cycles. In addition, myelosuppression occurred several times during chemotherapy, and grade IV myelosuppression occurred at the most severe stage, which mainly resulted in the reduction of leukocyte and neutrocyte count. The lowest leukocyte value was 0.9×109/L, the lowest neutrocyte value was 0.7×109/L. Recombinant human granulocyte colony-stimulating factor (rhG-CSF) was injected several times during the treatment. Thus, chemotherapy was discontinued on January 25, 2021, and the subsequent treatment regimen consisted of anlotinib combined with sintilimab once every 3 weeks. Since then, the regimen of anlotinib plus sintilimab has been maintained. A recent chest CT showed that the left lung mass was further reduced in size with a little inflammation in the left lung and right middle lung, and both lungs showed scattered foci of fibrosis. The axillary lymph nodes and subcutaneous nodules on both upper arms were also significantly smaller than before. The subcutaneous nodules and enlarged axillary lymph nodes were later not present on physical examination and were no longer shown on the CT scan. We believe that these 2 lesions were in complete remission (CR). At present, the patient continues to receive targeted therapy with anlotinib and immunotherapy with sintilimab and undergoes regular followed-up chest CT imaging and his disease remains in partial response (PR) (Figure 5). Up to now, the patient has no targeted and immunotherapy-related toxicities such as rash, hypertension, hepatorenal toxicity, cardiopulmonary toxicity, neurotoxicity, hand-foot syndrome, etc. However, a recent thyroid function test on May 04, 2022, showed that thyroidstimulatinghormone (TSH) was higher than 100 mIU/L, Free thyroid hormone 4 (FT4) was 6.58 pmol/L. And accompanied by obvious fatigue and hair loss. So, the diagnosis of immune-associated hypothyroidism grade 2 was considered. At the recommendation of the endocrinologist, the patient is currently taking Euthyrox for 300 µg qd and receiving regular thyroid function tests. TSH and FT4 values didn’t increase further. There was no serious toxicity (Grade 3 or 4) related to the targeted therapy and immunotherapy. All diagnosis, treatment, adverse reactions in the case are reflected in the way of timeline (Figure 6).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Due to the low incidence of PSC its’ treatment remains a challenge. Many therapeutic options suffer from poor efficacy and lack substantial clinical data to support them (15). In the past, inoperable advanced PSC has largely been treated with first-line platinum-based doublet chemotherapy. A retrospective analysis of the National Cancer Database showed that adjuvant chemotherapy was associated with an improved 5-year OS for stage II and stage III disease patients (16). However, Vieira et al.’s retrospective analysis showed that advanced PSC patients have a high degree of resistance to platinum-based chemotherapy with short OS (3). With advances in research on molecular mechanisms, PSC, similar to other NSCLC subtypes, has now entered a new era of targeted therapy and immunotherapy.
PSC is both morphologically and genetically diverse. Descriptions of driver gene mutations offer new hope for the development of personalized therapeutic options. The high expression of PD-L1 and MET mutations are relatively common in PSC (11,17). Immunotherapy efficacy seems to be corelated with PD-L1 expression in a retrospective study of 90 patients (12). Another publication of 39 patients with PSC treated with ICIs, mainly in the second-line, showed an ORR of 38.5%, a PFS of 4.6 months, and an OS 20 months (13). Several case reports have noted that combination therapy (with the MET inhibitor, crizotinib; ICIs namely, nivolumab, toripalimab, and camrelizumab; and VEGFR inhibitors namely anlotinib and apatinib) has promising efficacy in patients with various stages of PSC; and significantly improved the patients’ PFS and OS compared to the previous approach of surgery and chemotherapy, with some patients having achieved PR (6,18-22).
In our patient, the high PD-L1 expression of 70% is one of the important reasons for the favorable response to sintilimab. Sintilimab is a highly selective fully humanized immunoglobulin G4 (IgG4) monoclonal antibody that blocks the binding site of programmed cell death protein-1 (PD-1). It has been proven to be clinically beneficial in the treatment of multiple advanced solid tumors including NSCLC (23). To our knowledge, this is the first case in which sintilimab was used to treat PSC and in which a patient achieved a PR. Unfortunately, for financial reasons, the patient did not undergo genetic testing.
Antiangiogenic therapy may also benefit patients with PSC who are prone to vascular invasion. According to the Li et al., one PSC patient treated with apatinib, a TKI that selectively targets VEGFR2 expression in tumor cells, showed sustained tumor regression after treatment. No severe complications are associated with apatinib therapy (24). Patients with post-operative recurrent PSC treated with anlotinib combined with chemotherapy (dacarbazine and cisplatin) have also achieved CR (25). However, the effectiveness of targeted therapy with anlotinib is only based on limited case reports.
Several cases of combination therapy with antiangiogenic inhibitors and ICIs have also been reported (26-28). An adult male patient with high PD-L1 expression with a tumor proportion score of 90% and the Kirsten rat sarcoma viral oncogene (KRAS) exon 2 mutation was treated with nivolumab and anlotinib after a rapid recurrence of PSC during post-operative adjuvant chemotherapy. After 8 weeks, his clinical symptoms gradually resolved and his disease achieved a PR (27). In a phase 1b trial (NCT032628521) conducted at the Shanghai Chest Hospital, 22 advanced NSCLC patients treated with first-line sintilimab and anlotinib had a median ORR of 72.7% and a median DCR of 100% (28). Regardless of the histologic subtype or PD-L1 expression, this novel combination therapy has potential efficacy.
In general, despite previous studies showing that the median OS for patients with stage IV PSC is approximately 5.4 months, our patient who was treated with platinum-based chemotherapy, anlotinib, and sintilimab, achieved good local control with a long PR with no serious drug-related adverse events. Thus, with the provision that adverse events are manageable, this case suggests that combined platinum-based doublet chemotherapy, targeted therapy with an antiangiogenic agent, and immunotherapy with an ICI may result in favorable treatment outcomes in patients with advanced PSC.
Patient perspective
During the early phase of my disease, I experienced shortness of breath and chemotherapy induced nausea and vomiting. However, during the treatment with the combination of sintilimab and anlotinib, my performance status improved and I was able to engage in activities of daily living including performing household chores such as cleaning. I sometimes experienced fatigue which could be relieved by rest. At present, my symptoms related to the disease are well-controlled and I am able to tolerate further treatment.
Questions to be further discussed and considered
Question 1: What are the options for traditional chemotherapy for PSC? How effective are they?
Pierre Tankere: The previous authors already mentioned that traditional treatment for PSC include the same as in NSCLC: chemo with platinum-based chemo doublets (i.e., platinum + gemcitabine or pemetrexed or pacilitaxel of docetaxel) (29).
Chong-Kin Liam: PSC is not sensitive to first-line traditional cytotoxic chemotherapy agents. There is a lack of randomized controlled trials. First-line chemotherapy frequently includes pemetrexed, docetaxel, gemcitabine, paclitaxel, and vinorelbine.
Question 2: What is the progress of immunotherapy in the treatment of PSC cancer?
Pierre Tankere: According to the paper of Babacan et al. (12), among patients with advanced PSC, PD-L1 expression is significantly associated with increased tumor responses and improved PFS after checkpoint inhibitor immunotherapy. Another interesting paper of 39 pulmonary sarcomatoid cancer mainly 2nd line therapy showed ORR 38.5% and PFS of 4.6 months, OS of 20 months (13).
Chong-Kin Liam: PD-L1 positivity rate and expression level in PSC are high. It has previously been shown that PSC has a high tumor mutational burden and a T-cell-inflamed microenvironment. Two retrospective studies have shown preliminary antitumor effects of ICIs in patients having PSC with overall response rates of 40.5% (15/37) and 31.6% (6/19), respectively (13,30).
There is a correlation between PD-L1 expression and favorable tumor responses as well as PFS following immunotherapy with ICI (12).
So far, there are no treatment outcomes from prospective studies on PSC but some trials are ongoing. The combination of durvalumab (a PD-L1 inhibitor) and tremelimumab (a CTLA-4 inhibitor) has demonstrated effective antitumor activity with a manageable toxicity profile in locally advanced or metastatic NSCLC. The combination of durvalumab and tremelimumab in metastatic/relapsed PSC was investigated in a phase II single-arm, open-label, multicenter study (NCT03022500). The ORR and DCR in 15 evaluable subjects were 26.7% and 60.0%, respectively while the median PFS was 5.9 months and the median OS was 15.4 months (20).
Ongoing trials are investigating the efficacy of PD-1 inhibitors, toripalimab and camrelizumab (31,32).
There is preclinical and clinical evidence that the effect of ICI immunotherapy could be enhanced by low-dose apatinib (a VEGFR2 TKI). Low-dose apatinib has been shown to alleviate hypoxia, increase lymphocyte infiltration, and reduce tumor PD-L1 expression in a murine Lewis lung carcinoma model, thereby altering the tumor microenvironment from an immunosuppressive to a more permissive one for antitumor immunity (33). The combination of low-dose apatinib and anti-PD-L1 significantly slowed tumor growth, decreased the number of metastases, and lengthened survival of the mice.
In 105 nonsquamous NSCLC patients who had previous chemotherapy but immunotherapy-naïve, the ORR from a combination of apatinib plus camrelizumab was 27.6% (34).
Question 3: In this case, if the disease progresses, what subsequent treatment options are available?
Pierre Tankere: The treatment would of course depend on local habits and the clinical presentation. The options discussed would include pemetrexed, gemcitabine; vinorelbine. Given the unusual immunotherapy and antiangiogenic used in this case we could also challenge association with other molecules such as bevacizumab or pembrolizumab.
Chong-Kin Liam: If next-generation sequencing of the original tumor biopsy showed a targetable genetic alteration such as MET exon 14 skipping mutation, then a MET inhibitor such as crizotinib or capmatinib or tepotinib or savolitinib may be used for second-line treatment.
Question 4: In addition to the expression of PD-L1, are there other biomarkers for the immunotherapy of PSC?
Pierre Tankere: None that I know of has been precisely studied for PSC.
Chong-Kin Liam: Tumor mutational burden which tends to be high in PSC is another potential predictive biomarker. CD47 is a commonly expressed transmembrane glycoprotein which suppresses macrophage phagocytosis and aids the evasion of tumor cells from the immune system. CD47 expression may be another predictive biomarker. In PSC patients, the coexpression of PD-L1 and CD47 is a potential predictive biomarker for combination therapy with two ICIs (35).
Question 5: Are immunotherapy and antiangiogenic therapy alternatives to maintenance therapy for PSC? How long should maintenance treatment last?
Pierre Tankere: Same maintenance therapy as NSCLC can be discussed including pemetrexed. No clear consensus over duration of maintenance treatment as far as I know. Our habits include at least 2 years if well tolerated.
Chong-Kin Liam: A combination of immunotherapy and antiangiogenic therapy offers a well-tolerated option for maintenance therapy for PSC. Since, PSC is very rare it is unlikely that any clinical trial will be performed to determine the efficacy of this combination and the duration of maintenance treatment. In general, clinical trials for ICI therapy for advanced stage NSCLC is for up to 2 years. Due to the lack of clinical trial results, the experience with immunotherapy documented in case reports and real-world data may be the only source of evidence supporting this treatment approach.
Acknowledgments
The authors appreciate the academic support from AME Radiation Oncology Collaborative Group.
Funding: This work was supported by grants from the Minsheng Science and Technology Foundation of Suzhou (grant No. SYS2019037), and the Project of State Key Laboratory of Radiation Medicine and Protection, Soochow University (grant No. GZK1202002).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4312/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4312/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li X, Wu D, Liu H, et al. Pulmonary sarcomatoid carcinoma: progress, treatment and expectations. Ther Adv Med Oncol 2020;12:1758835920950207. [Crossref] [PubMed]
- Steuer CE, Behera M, Liu Y, et al. Pulmonary Sarcomatoid Carcinoma: An Analysis of the National Cancer Data Base. Clin Lung Cancer 2017;18:286-92. [Crossref] [PubMed]
- Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013;8:1574-7. [Crossref] [PubMed]
- Pécuchet N, Vieira T, Rabbe N, et al. Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities. Ann Oncol 2017;28:1597-604. [Crossref] [PubMed]
- Yang Z, Xu J, Li L, et al. Integrated molecular characterization reveals potential therapeutic strategies for pulmonary sarcomatoid carcinoma. Nat Commun 2020;11:4878. [Crossref] [PubMed]
- Han S, Fang J, Lu S, et al. Response and acquired resistance to savolitinib in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Onco Targets Ther 2019;12:7323-8. [Crossref] [PubMed]
- Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016;34:794-802. [Crossref] [PubMed]
- Miranda O, Farooqui M, Siegfried JM. Status of Agents Targeting the HGF/c-Met Axis in Lung Cancer. Cancers (Basel) 2018;10:280. [Crossref] [PubMed]
- Vieira T, Antoine M, Ruppert AM, et al. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014;85:276-81. [Crossref] [PubMed]
- Maneenil K, Xue Z, Liu M, et al. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin Lung Cancer 2018;19:e323-33. [Crossref] [PubMed]
- Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013;8:803-5. [Crossref] [PubMed]
- Babacan NA, Pina IB, Signorelli D, et al. Relationship Between Programmed Death Receptor-Ligand 1 Expression and Response to Checkpoint Inhibitor Immunotherapy in Pulmonary Sarcomatoid Carcinoma: A Pooled Analysis. Clin Lung Cancer 2020;21:e456-63. [Crossref] [PubMed]
- Domblides C, Leroy K, Monnet I, et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J Thorac Oncol 2020;15:860-6. [Crossref] [PubMed]
- Kong F, Wang W, Gong L, et al. Anti-PD-1 antibody camrelizumab plus doxorubicin showed durable response in pulmonary sarcomatoid carcinoma: Case report and literature review. J Clin Pharm Ther 2020;45:1489-96. [Crossref] [PubMed]
- Ung M, Rouquette I, Filleron T, et al. Characteristics and Clinical Outcomes of Sarcomatoid Carcinoma of the Lung. Clin Lung Cancer 2016;17:391-7. [Crossref] [PubMed]
- Abdallah HM, Martinez-Meehan D, Lutfi W, et al. Adjuvant chemotherapy for pulmonary sarcomatoid carcinoma: A retrospective analysis of the National Cancer Database. J Thorac Cardiovasc Surg 2022;163:1669-1681.e3. [Crossref] [PubMed]
- Pelosi G, Gasparini P, Conte D, et al. Synergistic Activation upon MET and ALK Coamplification Sustains Targeted Therapy in Sarcomatoid Carcinoma, a Deadly Subtype of Lung Cancer. J Thorac Oncol 2016;11:718-28. [Crossref] [PubMed]
- Jiao Y, Liu M, Luo N, et al. Successful treatment of advanced pulmonary sarcomatoid carcinoma with the PD-1 inhibitor toripalimab: A case report. Oral Oncol 2021;112:104992. [Crossref] [PubMed]
- Roesel C, Kambartel K, Kopeika U, et al. Lazarus-type tumour response to therapy with nivolumab for sarcomatoid carcinomas of the lung. Curr Oncol 2019;26:e270-3. [Crossref] [PubMed]
- Kim M, Keam B, Ock CY, et al. Phase II study of durvalumab and tremelimumab in pulmonary sarcomatoid carcinoma: KCSG-LU16-07. Thorac Cancer 2020;11:3482-9. [Crossref] [PubMed]
- Taniguchi H, Takemoto S, Ozasa M, et al. Remarkable response to pembrolizumab with platinum-doublet in PD-L1-low pulmonary sarcomatoid carcinoma: A case report. Thorac Cancer 2021;12:1126-30. [Crossref] [PubMed]
- Wang X, Cao J, Du W, et al. Response to gefitinib/crizotinib combination in a pulmonary sarcomatoid carcinoma patient harboring concurrent EGFR mutation and MET amplification. Clin Case Rep 2021;9:e04487. [Crossref] [PubMed]
- Liu X, Yi Y. Recent updates on Sintilimab in solid tumor immunotherapy. Biomark Res 2020;8:69. [Crossref] [PubMed]
- Li X, He Y, Zhu J, et al. Apatinib-based targeted therapy against pulmonary sarcomatoid carcinoma: a case report and literature review. Oncotarget 2018;9:33734-8. [Crossref] [PubMed]
- Li J, Liang H, He J, et al. Anlotinib Combined With Chemotherapy for Recurrence of Pulmonary Sarcomatoid Cancer Previously Surgically Treated: A Case Report and Literature Review. Front Oncol 2021;11:639168. [Crossref] [PubMed]
- Luo Y, Wei J, Zhang J, et al. Two different patients with pulmonary pleomorphic carcinoma response to PD-1 inhibitor plus anlotinib. Lung Cancer 2021;155:170-4. [Crossref] [PubMed]
- Jin C, Yang B. Dramatic Response of Pulmonary Sarcomatoid Carcinoma to Nivolumab Combined with Anlotinib: A Case Report. Case Rep Oncol 2020;13:601-5. [Crossref] [PubMed]
- Chu T, Zhong R, Zhong H, et al. Phase 1b Study of Sintilimab Plus Anlotinib as First-line Therapy in Patients With Advanced NSCLC. J Thorac Oncol 2021;16:643-52. [Crossref] [PubMed]
- Karim NA, Schuster J, Eldessouki I, et al. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget 2018;9:4102-8. [Crossref] [PubMed]
- Manglaviti S, Brambilla M, Signorelli D, et al. Immune-Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer With Uncommon Histology. Clin Lung Cancer 2022;23:e17-e28. [Crossref] [PubMed]
- U.S. National Library of Medicine. Clinical trials on pulmonary sarcomatoid carcinoma[Internet]. Bethesda: U.S. National Library of Medicine; 2021 [cited 2022 Jul 9]. Available online: https://clinicaltrials.gov/ct2/results?cond=pulmonary+sarcomatoid+carcinoma& term=&cntry=&state=&city=&dist=
- Chinese Clinical Trial Registry. Clinical Trials on Pulmonary Sarcomatoid Carcinoma[Internet]. 2021 [cited 2022 Jul 9]. Available online: https://www.chictr.org.cn/
- Zhao S, Ren S, Jiang T, et al. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res 2019;7:630-43. [Crossref] [PubMed]
- Zhou C, Wang Y, Zhao J, et al. Efficacy and Biomarker Analysis of Camrelizumab in Combination with Apatinib in Patients with Advanced Nonsquamous NSCLC Previously Treated with Chemotherapy. Clin Cancer Res 2021;27:1296-304. [Crossref] [PubMed]
- Yang Z, Xu J, Li R, et al. PD-L1 and CD47 co-expression in pulmonary sarcomatoid carcinoma: a predictor of poor prognosis and potential targets of future combined immunotherapy. J Cancer Res Clin Oncol 2019;145:3055-65. [Crossref] [PubMed]


