
Bergenin inhibits palmitic acid-induced pancreatic β-cell inflammatory death via regulating NLRP3 inflammasome activation
Introduction
Diabetes mellitus is a rising health burden globally with a high prevalence. More than 400 million patients suffered from diabetes mellitus in 2014 and the number of diabetes mellitus patients will increase to 600 million in 2035 (1). Type 2 diabetes mellitus (T2DM) accounts for 90% of all cases of diabetes mellitus and is characterized by insulin resistance, pancreatic islet failure, and a deficit in pancreatic β-cell mass and function (2). The risk factors for T2DM are diverse, including body weight, circulating free fatty acids, metabolic syndrome, lifestyle, sleep deprivation, smoking, work stress, and alcohol, among others (3,4). Patients with T2DM are at risk for cardiovascular disease, stroke, diabetic foot syndrome, and renal insufficiency, making it the 9th major cause of death worldwide (5,6). The main goals of T2DM management currently are insulin regulation and complication prevention (7,8). Life-long insulin in combination with antidiabetic drugs, such as metformin and glimepiride, is the standard treatment for T2DM (8).
Bergenin is an isocoumarin compound isolated from many plant species, including genus Bergenia, which has been recognized as a traditional herbal medicine in Asia for treating cough and kidney stone (9). Bergenin shows a wide array of pharmacological activities and has antioxidant (10), anti-inflammatory (11), anti-arrhythmic (12), anti-fibrotic (13), and anti-cancer properties (14). Bergenin plays an anti-inflammatory role via inhibiting the activation of the NF-κB and MAPK signaling pathways in lipopolysaccharide-induced mastitis (15). Bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-κB activation (16). Moreover, bergenin exerts antidiabetic activity via promoting pancreatic β-cell regeneration (17) and preventing cytokine-induced apoptosis (18). Bergenin has also been reported to be beneficial in treating diabetic complications, including diabetic nephropathy (19,20) and retinopathy (21). However, the effects of bergenin on regulating inflammatory pathways in T2DM remain largely unknown.
NOD-like receptor family-pyrin domain containing 3 (NLRP3) inflammasome, composed of NLRP3 protein, caspase-1, and apoptosis associated speck like protein (ASC), plays a role in the maturation of the proinflammatory cytokines interleukin (IL)-1β and IL-18 and the induction of pyroptosis (22). NLRP3 affects pathogenic T-cell migration to the pancreatic islet (23), and is associated with the development and progression of T2DM (24). In addition, the activation of NLRP3 inflammasome is associated with obesity-induced insulin resistance (25). Bergenin has been reported to be an anti-inflammatory agent in ameliorating the development of 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced acute colitis in rats via blocking NLRP3 inflammasome signaling (26).
In this study, we aimed to demonstrate the effects of bergenin on NLRP3 inflammasome-related inflammation in pancreatic β-cell lines. Several in vitro experiments were performed using INS-1 and MIN6 cells and the results will enlarge our understanding of the antidiabetic effect of bergenin. We present the following article in accordance with the MDAR reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3781/rc).
Methods
Pancreatic β-cell culture
INS-1 (catalog number: CL-0368) and MIN6 (catalog number: CL-0674) cells were purchased from Procell (Wuhan, China) and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Procell) with 10% fetal bovine serum (Hyclone, Logan, UT, USA) and 0.05 mM β-mercaptoethanol (Sigma, St. Louis, MO, USA) in a 5% CO2 incubator at 37 ℃.
Palmitic acid (PA; Sigma) was dissolved in 90% ethanol and diluted in RPMI-1640 medium. Cells were treated with PA for 48 h with a final concentration of 400 µM. Cells were treated with 1, 3, or 10 µM bergenin (Chengdu Must Bio-technology Co., Ltd., Chengdu, China) in the absence or presence of PA for 48 h (20).
Cell transfection
NLRP3 overexpression plasmid was constructed and purchased from Shanghai Yaji Biotechnology Co., Ltd. (Shanghai, China). For cell transfection, 1.5 µg of NLRP3 overexpression plasmid or the empty pcDNA3.1 vector was added into 50 µL of culture medium, and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used in this procedure. At 48 h later, cells were collected for the subsequent experiments.
Cell viability
The viability of INS-1 and MIN6 cells was detected using Cell Counting Kit (CCK)-8 (Dojindo Molecular Technologies, Japan). Cells in 96-well plates (5,000 cells/well) were treated with bergenin and PA for the indicated time, then 10 µL CCK-8 reagent was added for a 4 h incubation at 37 ℃. The absorbance was read using a microplate detector (Bio-Rad, Hercules, CA, USA) at 450 nm and the percentage of cell survival was calculated.
Flow cytometry
The apoptosis of INS-1 and MIN6 cells was detected using an Annexin V-FITC apoptosis detection kit (Beyotime, Shanghai, China). Following treatment with bergenin and PA for the indicated time, cells in 24-well plates were collected. Cells were collected in binding buffer with a density of 5×105 cells per sample and stained with 10 µL Annexin V-FITC and 5 µL PI for 30 min in the dark. Annexin V-FITC-labeled cells were counted using a flow cytometer (BD Biosciences, San Jose, CA, USA) as apoptotic cells.
qRT-PCR
Cellular RNA was extracted using the RNAprep Pure Cell/Bacteria Kit (TIANGEN, Beijing, China). The real-time PCR procedure was performed using the FastKing One-Step Reverse Transcription-Fluorescent Quantification Kit (TIANGEN). The primer sequences used are listed in Table 1.
Table 1
| Gene name | Primer sequences |
|---|---|
| Rattus norvegicus IL-6 | 5'-AGAGACTTCCAGCCAGTTGC-3' (forward) |
| 5'-TGCCATTGCACAACTCTTTTC-3' (reverse) | |
| Rattus norvegicus TNF-α | 5'-ATGGGCTCCCTCTCATCAGT-3' (forward) |
| 5'-GCTTGGTGGTTTGCTACGAC-3' (reverse) | |
| Rattus norvegicus IL-1β | 5'-GGGCCTCAAGGGGAAGAATC-3' (forward) |
| 5'-TTTGGGATCCACACTCTCCAG-3' (reverse) | |
| Rattus norvegicus IL-18 | 5'-CAGCTCTTCTACCAGCAAACAT-3' (forward) |
| 5'-CTTCCAACTGAGAGGCTGTGC-3' (reverse) | |
| Rattus norvegicus β-actin | 5'-CCGCGAGTACAACCTTCTTG-3' (forward) |
| 5'-CGTCATCCATGGCGAACTGG-3' (reverse) | |
| Mus musculus IL-6 | 5'-GCCTTCTTGGGACTGATGCT-3' (forward) |
| 5'-TGTGACTCCAGCTTATCTCTTGG-3' (reverse) | |
| Mus musculus TNF-α | 5'-ACCCTCACACTCACAAACCA-3' (forward) |
| 5'-GAGGCAACCTGACCACTCTC-3' (reverse) | |
| Mus musculus IL-1β | 5'-TGCCACCTTTTGACAGTGATG-3' (forward) |
| 5'-TTCTTGTGACCCTGAGCGAC-3' (reverse) | |
| Mus musculus IL-18 | 5'-CCCTTTGAGGCATCCAGGAC-3' (forward) |
| 5'-GGGGTTCACTGGCACTTTGA-3' (reverse) | |
| Mus musculus β-actin | 5'-CCAGCCTTCCTTCTTGGGTAT-3' (forward) |
| 5'-GGGTGTAAAACGCAGCTCAG-3' (reverse) |
Western blot
Total cellular proteins were prepared using ice-cold RIPA buffer containing 1% halt protease inhibitor (Beyotime). Proteins (50 µg/sample) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% non-fat milk followed by incubation with primary anti-IL-6 (ab214429), anti-TNF-α (ab183218), anti-IL-1β (ab216995), anti-IL-18 (ab207324), anti-NLRP3 (ab270449), anti-ASC (ab155970), anti-GSDMD-N (ab215203), anti-β-actin (ab8226) (1:1,000, Abcam, USA), and anti-cleaved caspase-1 (#89332) (1:1,000, Cell Signaling Technology, USA) antibodies at 4 ℃ overnight. The membranes were washed and incubated with anti-mouse (ab97040) and anti-rabbit (ab7090) HRP secondary antibodies (1:2,000, Abcam), and the protein bands were detected with enhanced chemiluminescence (Bio-Rad).
Immunofluorescence
Cells were grown on glass coverslips in 60 mm cell culture dishes. Following 3 washes with phosphate buffered saline (PBS), cells on glass coverslips were fixed with 4% paraformaldehyde for 20 min, permeabilized in 0.2% Triton X100 for 10 min, and incubated with primary anti-NLRP3 antibody (PA5-18118) (1:50, Invitrogen, USA) at 4 ℃ overnight. After incubation with the fluorescence secondary antibody (A-11055) (1:200, Invitrogen, USA), the nuclei were stained with DAPI. The NLRP3-labelled cells were captured by a fluorescence microscope (IX73; Olympus, Tokyo, Japan).
Molecular docking
SwissTargetPrediction (http://www.swisstargetprediction.ch/) and Comparative Toxicogenomics Database (CTD; https://ctdbase.org/) were used to predict the potential targets of bergenin. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed to screen bergenin-regulated signaling pathways which are associated with diabetes mellitus. The molecular docking analysis between bergenin and NLRP3 (identifier: 2naq) was performed using Autodock Vina software and the interaction results were measured using Discovery Studio 4.5 Client software. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
Results are presented as mean ± standard deviation (SD) from 3 independent experiments. Data was analyzed using GraphPad Prism 7.0 software (La Jolla, CA, USA). Statistical analyses were performed using one- or two-way analysis of variance (ANOVA) with Tukey’s post-hoc test. P values less than 0.05 were considered as statistically significant.
Results
Bergenin inhibits PA-induced pancreatic β-cell apoptosis
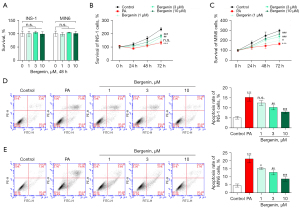
Two pancreatic β-cell lines, INS-1 and MIN6, were treated with 1, 3, or 10 µM bergenin for 48 h and cell survival was measured by CCK-8 kits. In contrast to the non-treated cells, bergenin had no significant impact on the survival rate of treated cells (P>0.05, Figure 1A), indicating that bergenin with final concentrations less than or equal to 10 µM had no cytotoxicity in INS-1 and MIN6 cells. INS-1 and MIN6 cells were then treated with bergenin in the absence or presence of 400 µM PA for 48 h. Data in Figure 1B,1C showed that cells subjected to PA treatment exhibited significantly decreased cell survival (P<0.05). Bergenin treatment increased cell survival in a dose-dependent manner (P<0.05). In addition, the apoptosis rate of INS-1 and MIN6 cells was high in the PA-treated group (P<0.05, Figure 1D,1E). Bergenin treatment dose-dependently decreased PA-induced apoptosis. Bergenin with a final concentration of 10 µM reduced the apoptosis rate from 15.2% to 7.9% in INS-1 cells and from 21.1% to 8.6% in MIN6 cells.
Bergenin inhibits PA-induced pancreatic β-cell inflammation
We next examined the effects of bergenin on PA-induced cytokine release in pancreatic β-cells. qRT-PCR data in Figure 2A-2H showed that the mRNA levels of IL-6, TNF-α, IL-1β, and IL-18 in PA-treated INS-1 and MIN6 cells were higher than those in non-treated controls (P<0.05). Treating cells with bergenin significantly decreased cytokine release in a dose-dependent manner (P<0.05). In addition, the protein levels of these cytokines were increased by PA and decreased with increasing doses of bergenin (Figure 2I,2J).

Bergenin inhibits PA-induced NLRP3 inflammasome activation in pancreatic β-cells
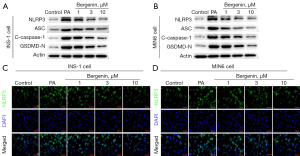
The activation of the NLRP3 inflammasome in pancreatic β-cells was analyzed to study whether bergenin exerted anti-inflammatory properties via regulating NLRP3. It was shown that the levels of NLRP3, ASC, cleaved caspase-1, and GSDMD-N proteins were notably increased by PA and decreased with increasing doses of bergenin (Figure 3A,3B). Immunofluorescent staining of NLRP3 confirmed the inhibitory effects of bergenin on NLRP3 protein expression (Figure 3C,3D).
Bergenin inhibits NLRP3 expression via regulating its protein stability
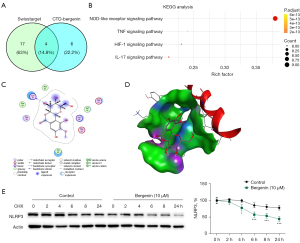
We then studied whether bergenin decreased NLRP3 expression in pancreatic β-cells. SwissTargetPrediction and CTD databases screened 4 pathways which are downstream signaling pathways of bergenin (Figure 4A) and identified NOD-like receptor, TNF, HIF-1, and IL-17 signaling pathways (Figure 4B). NLRP3 has long been known as an effector of the NOD-like receptor signaling pathway in regulating various inflammatory diseases, including diabetes mellitus (27). Next, we used Autodock Vina software to dock bergenin with NLRP3 protein. Molecular docking results were shown in 2D (Figure 4C) and 3D views (Figure 4D). The binding energy of interaction was −5.101 kcal/mol and the RMSD score was 1.5901A. By adding 100 µM cycloheximide (CHX) into the cell culture medium, the degradation of NLRP3 was analyzed. NLRP3 protein in the control group degraded slowly, but degraded rapidly in bergenin-treated cells (P<0.05, Figure 4E), indicating that bergenin targets NLRP3 and accelerates its degradation.
Bergenin inhibits PA-induced MIN6 cell apoptosis and inflammation via regulating NLRP3
To study whether bergenin has anti-apoptotic and anti-inflammatory properties in pancreatic β-cells via inactivation of NLRP3, MIN6 cells were transfected with NLRP3 overexpression plasmid. The transfection efficiency was confirmed by analyzing NLRP3 protein expression using western blotting analysis (Figure 5A). The overexpression plasmids reversed the downregulation of NLRP3 in bergenin-treated cells as expected (Figure 5B). In contrast to the cells transfected with empty vector, transfection of cells with NLRP3 overexpression plasmids significantly reversed the promotive effects of bergenin on the MIN6 cell survival rate (P<0.05, Figure 5C). In addition, in contrast to empty vector transfection, NLRP3 overexpression plasmids significantly reversed the inhibitory effects of bergenin on MIN6 cell apoptosis and cytokine levels (P<0.05, Figures 5D,6A-6E).


Discussion
The loss of pancreatic β-cell mass and function is a main characteristic of T2DM (2). In this study, 2 pancreatic β-cell lines, INS-1 and MIN6, were subjected to PA to mimic in vitro models of T2DM as previously reported (28). As expected, PA, a kind of free fatty acid, significantly reduced pancreatic β-cell survival, induced apoptosis, and initiated NLRP3 inflammasome-related inflammation. Bergenin with concentrations of 1, 3, and 10 µM attenuated PA-induced pancreatic β-cell loss and inflammation. In addition, NLRP3 was predicted and confirmed as a target protein of bergenin. Bergenin inhibited NLRP3 expression via directly promoting its protein degradation. Furthermore, restoration of NLRP3 expression using plasmid transfection reversed the protective effects of bergenin on pancreatic β-cells.
Recently, a number of traditional herbal medicines have been identified to have antidiabetic properties in T2DM (29). Ginsenoside Rg1 (30), Rc (31), Rk3 (32), and Rb1 (33) are able to repress endothelial and pancreatic β-cell apoptosis and inflammation and are beneficial in preventing diabetic complications, such as diabetic nephropathy and retinopathy. Active ingredients extracted from Astragalus membranaceus, including baicalin, baicalein, and wogonin, relieved high glucose-induced endothelial inflammation to prevent and treat diabetic complications (34). Treating rats with protocatechualdehyde effectively relieved streptozotocin-induced endothelial dysfunction (35). In this study, we demonstrated the anti-apoptotic and anti-inflammatory properties of bergenin in pancreatic β-cells, suggesting it as a potential agent for treating T2DM.
The antidiabetic activity of bergenin has been identified in recent years. Bergenin protected pancreatic β-cells against cytokine-induced apoptotic cell death and the dysfunction of insulin secretion (18). Diabetic rats administered with bergenin exhibited lower fasting blood glucose, plasma lipid profiles, and oxidative stress (17). In addition, the potential of bergenin to treat diabetic complications has been evaluated. Bergenin is beneficial in ameliorating diabetic nephropathy (20), neuropathy (19), and retinopathy (21) through inhibition of the TGF-β1/Smads and mTOR/β-TrcP/Nrf2 signaling pathways. However, we first demonstrated that bergenin inhibited pancreatic β-cell inflammation through inhibiting the activation of the NLRP3 inflammasome, as evidenced by the significant downregulation of NLRP3, ASC, cleaved caspase-1, and GSDMD-N as well as the reduced levels of cytokines.
The activation of the NLRP3 inflammasome plays a significant role in the development of T2DM (23,24). A human study characterized that the NLRP3 inflammasome was activated in the early stage of plaque formation in patients with T2DM and carotid atherosclerosis (36). Inhibition of NLRP3 expression directly via gene silencing (37) or indirectly via SGLT2 inhibitors, such as dapagliflozin (38) and empagliflozin (39), is effective in ameliorating insulin resistance and diabetic complications. In addition, various active ingredients of traditional herbal medicine, such as shionone (40), crocin (41), morinda officinalis oligosaccharides (42), and dehydrocostus lactone (43), exerted anti-inflammatory property via inhibition of NLRP3 inflammasome. In this study, we demonstrated that bergenin is a novel inhibitor of the NLRP3 inflammasome as it effectively inhibited NLRP3 expression via promoting the degradation of NLRP3 in pancreatic β-cells. Furthermore, in vitro studies suggested that bergenin exerted its antidiabetic effects through inhibiting the activation of the NLRP3 inflammasome. The regulation of bergenin on NLRP3 inflammasome activation has been previously reported in rats with acute colitis (26). For the first time, this study linked bergenin with the NLRP3 inflammasome in T2DM in vitro. Further studies are required to confirm this hypothesis in animal models of T2DM. The potential application value and dosage safe of bergenin in clinical practices also need to be further studied.
In conclusion, our study showed the anti-apoptotic and anti-inflammatory properties of bergenin in PA-induced pancreatic β-cells. The beneficial effects of bergenin may be due to its regulation of NLRP3 inflammasome activation. This study suggests bergenin is a potential agent for the treatment of T2DM.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3781/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3781/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3781/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137-49. [Crossref] [PubMed]
- Javeed N, Matveyenko AV. Circadian Etiology of Type 2 Diabetes Mellitus. Physiology (Bethesda) 2018;33:138-50. [Crossref] [PubMed]
- Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev 2016;37:278-316. [Crossref] [PubMed]
- Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia 2020;63:2359-71. [Crossref] [PubMed]
- Cohen R, Sforza NS, Clemente RG. Impact of Metabolic Surgery on Type 2 Diabetes Mellitus, Cardiovascular Risk Factors, and Mortality: A Review. Curr Hypertens Rev 2021;17:159-69. [Crossref] [PubMed]
- Kelsey MD, Nelson AJ, Green JB, et al. Guidelines for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: JACC Guideline Comparison. J Am Coll Cardiol 2022;79:1849-57. [Crossref] [PubMed]
- Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol 2021;17:484-95. [Crossref] [PubMed]
- Pfeiffer AF, Klein HH. The treatment of type 2 diabetes. Dtsch Arztebl Int 2014;111:69-81; quiz 82. [PubMed]
- Koul B, Kumar A, Yadav D, et al. Bergenia Genus: Traditional Uses, Phytochemistry and Pharmacology. Molecules 2020;25:5555. [Crossref] [PubMed]
- Zhang G, Wang H, Zhang Q, et al. Bergenin alleviates H2O2-induced oxidative stress and apoptosis in nucleus pulposus cells: Involvement of the PPAR-γ/NF-κB pathway. Environ Toxicol 2021;36:2541-50. [Crossref] [PubMed]
- Deng L, Song C, Niu Y, et al. Synthesis and biological evaluation of bergenin derivatives as new immunosuppressants. RSC Med Chem 2021;12:1968-76. [Crossref] [PubMed]
- Pu HL, Huang X, Zhao JH, et al. Bergenin is the antiarrhythmic principle of Fluggea virosa. Planta Med 2002;68:372-4. [Crossref] [PubMed]
- Li X, Wang Y, Liang J, et al. Bergenin attenuates bleomycin-induced pulmonary fibrosis in mice via inhibiting TGF-β1 signaling pathway. Phytother Res 2021;35:5808-22. [Crossref] [PubMed]
- Liu J, Zhang Y, Yu C, et al. Bergenin inhibits bladder cancer progression via activating the PPARγ/PTEN/Akt signal pathway. Drug Dev Res 2021;82:278-86. [Crossref] [PubMed]
- Gao XJ, Guo MY, Zhang ZC, et al. Bergenin Plays an Anti-Inflammatory Role via the Modulation of MAPK and NF-κB Signaling Pathways in a Mouse Model of LPS-Induced Mastitis. Inflammation 2015;38:1142-50. [Crossref] [PubMed]
- Yang S, Yu Z, Wang L, et al. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J Ethnopharmacol 2017;200:147-55. [Crossref] [PubMed]
- Kumar R, Patel DK, Prasad SK, et al. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia 2012;83:395-401. [Crossref] [PubMed]
- Rajput SA, Mirza MR, Choudhary MI. Bergenin protects pancreatic beta cells against cytokine-induced apoptosis in INS-1E cells. PLoS One 2020;15:e0241349. [Crossref] [PubMed]
- Villarreal CF, Santos DS, Lauria PSS, et al. Bergenin Reduces Experimental Painful Diabetic Neuropathy by Restoring Redox and Immune Homeostasis in the Nervous System. Int J Mol Sci 2020;21:4850. [Crossref] [PubMed]
- Qiao S, Liu R, Lv C, et al. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/β-TrcP/Nrf2 pathway. Free Radic Biol Med 2019;145:118-35. [Crossref] [PubMed]
- Yin Y, Xu R, Ning L, et al. Bergenin alleviates Diabetic Retinopathy in STZ-induced rats. Appl Biochem Biotechnol 2022; Epub ahead of print. [Crossref] [PubMed]
- Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol 2021;18:2114-27. [Crossref] [PubMed]
- Hu C, Ding H, Li Y, et al. NLRP3 deficiency protects from type 1 diabetes through the regulation of chemotaxis into the pancreatic islets. Proc Natl Acad Sci U S A 2015;112:11318-23. [Crossref] [PubMed]
- Chen X, Zhang D, Li Y, et al. NLRP3 inflammasome and IL-1β pathway in type 2 diabetes and atherosclerosis: Friend or foe? Pharmacol Res 2021;173:105885. [Crossref] [PubMed]
- Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179-88. [Crossref] [PubMed]
- Lopes de Oliveira GA, Alarcón de la Lastra C, Rosillo MÁ, et al. Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem Biol Interact 2019;297:25-33. [Crossref] [PubMed]
- Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141-50. [Crossref] [PubMed]
- Huang JS, Guo BB, Wang GH, et al. DGAT1 inhibitors protect pancreatic β-cells from palmitic acid-induced apoptosis. Acta Pharmacol Sin 2021;42:264-71. [Crossref] [PubMed]
- Xu YXZ, Xi S, Qian X. Evaluating Traditional Chinese Medicine and Herbal Products for the Treatment of Gestational Diabetes Mellitus. J Diabetes Res 2019;2019:9182595. [Crossref] [PubMed]
- Alolga RN, Nuer-Allornuvor GF, Kuugbee ED, et al. Ginsenoside Rg1 and the control of inflammation implications for the therapy of type 2 diabetes: A review of scientific findings and call for further research. Pharmacol Res 2020;152:104630. [Crossref] [PubMed]
- Wang Y, Fu W, Xue Y, et al. Ginsenoside Rc Ameliorates Endothelial Insulin Resistance via Upregulation of Angiotensin-Converting Enzyme 2. Front Pharmacol 2021;12:620524. [Crossref] [PubMed]
- Liu Y, Deng J, Fan D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct 2019;10:2538-51. [Crossref] [PubMed]
- He JY, Hong Q, Chen BX, et al. Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol Sin 2022;43:342-53. [Crossref] [PubMed]
- Ku SK, Bae JS. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep 2015;48:519-24. [Crossref] [PubMed]
- Ji B, Yuan K, Li J, et al. Protocatechualdehyde restores endothelial dysfunction in streptozotocin-induced diabetic rats. Ann Transl Med 2021;9:711. [Crossref] [PubMed]
- Lee J, Wan J, Lee L, et al. Study of the NLRP3 inflammasome component genes and downstream cytokines in patients with type 2 diabetes mellitus with carotid atherosclerosis. Lipids Health Dis 2017;16:217. [Crossref] [PubMed]
- Luo B, Li B, Wang W, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One 2014;9:e104771. [Crossref] [PubMed]
- Ye Y, Bajaj M, Yang HC, et al. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc Drugs Ther 2017;31:119-32. [Crossref] [PubMed]
- Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 2020;11:2127. [Crossref] [PubMed]
- Wang X, Yin H, Fan L, et al. Shionone alleviates NLRP3 inflammasome mediated pyroptosis in interstitial cystitis injury. Int Immunopharmacol 2021;90:107132. [Crossref] [PubMed]
- Zhang L, Previn R, Lu L, et al. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res Bull 2018;142:352-9. [Crossref] [PubMed]
- Li Z, Xu H, Xu Y, et al. Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation. CNS Neurosci Ther 2021;27:1570-86. [Crossref] [PubMed]
- Chen Y, Li R, Wang Z, et al. Dehydrocostus lactone inhibits NLRP3 inflammasome activation by blocking ASC oligomerization and prevents LPS-mediated inflammation in vivo. Cell Immunol 2020;349:104046. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


