Inhibitory effects of isoliquiritin on an atopic dermatitis model through the CD177/JAK2/STAT pathway in vitro and in vivo
Introduction
Atopic dermatitis (AD), a chronic, inflamed skin condition, is characterized by recurring eczematous lesions, intense pruritus, markedly increased immune cell numbers (mast cells, eosinophils, lymphocytes), and high levels of inflammatory mediators such as thymic stromal lymphopoietin (TSLP), interleukin (IL)-4, and immunoglobulin (IgE) in serum (1,2). In general, AD has seasonal variations. The prevalence of AD is 2.0–7.3% in adults and 20% in children (3-5). Several studies have indicated that AD is the skin manifestation of systemic disease and food allergies, hidradenitis suppurativa, asthma, conjunctivitis, or allergic rhinitis (6-10). With its high prevalence, comorbidities, and disease-related disabilities, AD imposes a considerable burden on global public health (5,11).
AD pathogenesis is multifactorial and complex. It involves genetic changes, skin-barrier disorders, skin disturbance due to microbes, as well as an imbalance in the number of T helper type 1 (Th1) and/or Th2 cells. Studies have shown that a T cell-mediated immune response can increase the secretion of proinflammatory cytokines. This action results in the activation and proliferation of mast cells and other immune cells, which leads to further T-cell activation. (12,13). In particular, Th2 cells have been shown to be involved in disease progression in AD patients in Asia and Europe (1). Th2 cells release cytokines (e.g., IL-4) resulting in the activation and proliferation of mast cells and eosinophils (14).
The human mast cell line HMC1.1 carrying a mutation in cluster of differentiation 177 (CD177; C-KIT V560G) has been shown to lead to the spontaneous proliferation and activation of cells coupled to the Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway (15,16).
Topical or systemic treatments with anti-inflammatory or immunosuppressive agents (such as corticosteroids, methotrexate and calcineurin inhibitors) are usually employed to alleviate AD symptoms (17-19), but do not lead to a cure. Moreover, several negative side effects are associated with the topical application of corticosteroids or calcineurin inhibitors, including erythema, papules, pustules, repeated flares of photosensitivity, and intense burning and itching (18,20). This limits their use as long-term therapies and leads to a poor quality of life, increased economic burden, and reduced work productivity.
Research has shown that natural ingredients with few side effects can be employed to treat AD. There have been several studies demonstrating the effectiveness of Chinese medicine formulations in treating AD for many years (21-26). The Chinese medicine formulation Pei Tu Qing Xin (PTQX) has been investigated in AD treatment for over a decade. PTQX has demonstrated potent anti-AD, anti-inflammatory, immunomodulatory, and antipruritic efficacy in moderate-to-severe AD patients in assessor-blinded, placebo-controlled, three-arm randomized clinical trials (27-29).
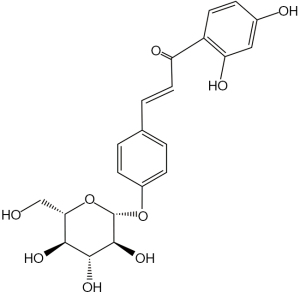
ISO a flavonol glycoside contained in licorice (Glycyrrhiza glabra), is an essential biologically active ingredient of PTQX (30). ISO (Figure 1) has been reported to possess anti-hyperalgesic, antioxidant, anti-inflammatory, immunomodulatory, anti-cancer, anti-depression, antifungal, wound-healing, and antiallodynic effects (31-37). These effects may play a substantial role in modulating AD’s pathological mechanism. It is unclear, however, whether ISO can be used to treat AD (and, if so, what mechanisms underlie the effect of ISO). In addition, the effect of ISO on AD has not been reported.
Therefore, we investigated ISO’s effect and explored its potential underlying molecular mechanisms in AD. A benzene derivative, 1-chloro-2,4-dinitrochlorobenzene (DNCB), which is highly electrophilic and cytotoxic, can induce clinical conditions similar to AD in mice (38,39). The aim of this study was to test the inhibitory effects of ISO on AD in NC/Nga mice induced by DNCB, and at the same time, test its effects in the human mast cell line HMC1.1 treated by calcium ionophore A23187/phorbol-12-myristate-13-acetate (PMA). We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3989/rc).
Methods
Reagents
ISO (Chemical Abstracts Service number: 5041-81-6; purity ≥98%) was obtained from Chengdu Must Bio-Technology (Chengdu, China). We dissolved ISO in dimethyl sulfoxide (DMSO) at a concentration of 100 mM, and we further diluted it in culture medium before each experiment in vitro. As a precaution against cytotoxicity, DMSO was diluted to less than 0.1% in all experiments. From Gibco (Grand Island, NY, USA), we purchased Iscove’s modified Dulbecco’s medium (IMDM), phosphate-buffered saline (PBS), penicillin, fetal bovine serum (FBS), and streptomycin. DNCB (purity ≥99%), calcium ionophore A23187, DMSO, polyvinylidene difluoride (PVDF) membranes (0.45 µm), PMA, and dexamethasone (DEX) were acquired from Millipore Sigma (Burlington, MA, USA).
Multi Sciences Biotech (Hangzhou, China) provided us with an annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit. We used the Cell Counting Kit 8 (CCK8) and RIPA buffer both from Beyotime Institute of Biotechnology (Shanghai, China). An assay kit for bicinchoninic acid (BCA) was obtained from Thermo Fisher Scientific.
Mouse ELISA kits for IL-4 (RAB0299) and IL-6 (RAB0308) were from Merck Millipore (Waltham, MA, USA). An IgE ELISA kit (catalog number: ab157718) and TSLP ELISA kit (ab155461) were purchased from Abcam (Cambridge, MA, USA). In addition, ELISA kits for IL-6 (ab178013) and IL-8 (ab46032) for cell supernatants were obtained from Abcam.
From Cell Signaling Technology, we procured antibodies against phosphorylated (p)-STAT1 (Tyr701), p-STAT5 (Y694), p-C-KIT (Tyr703), p-STAT3 (Tyr705), STAT3, STAT1, STAT5, BAX, and cleaved caspase-3. Antibodies against p-JAK2 were obtained from Abcam. We obtained a BCA assay kit from Thermo Fisher Scientific (Waltham, MA, USA).
Culture and stimulation of cells
Both of Millipore Sigma’s mast cell lines, HMC1.1 (KIT V560G mutation) and HMC1.2 (KIT V560G and D816V mutations), are derived from human mast cells. A humidified incubator maintained at 37 ℃ with 5% CO2 was used to maintain human mast cell lines. For cell culture, IMDM containing 1% (v/v) penicillin/streptomycin and 10% (v/v) FBS was used as the medium. Following preincubations with or without ISO for 2 h, calcium ionophore A23187 (1 mM) and PMA (0.05 mM) were added.
Cell viability
After plating into 96-well plates, HMC1.1 cells (1.0×104/200 µL) and HMC1.2 cells (1.0×104/200 µL) were cultured in a 37 ℃ incubator for 24 h with various concentrations of ISO. For the CCK8 assay, HMC1.1 cells in 96-well plates underwent addition with CCK8 solution (10 µL) and were incubated for an additional 1 h at 37 ℃. The optical density (OD) at 490 nm in 96-well plates was measured using a microplate reader (SpectraMax™ M2; Molecular Devices, Silicon Valley, CA, USA). The OD of untreated control cells was taken to indicate 100% viability. Relative cell viability was calculated using the formula ODsample/ODcontrol × 100%.
Flow cytometry
In HMC1.1 cells, we detected apoptosis using an annexin V-FITC/PI apoptosis kit. After harvesting, washing, and collecting cells in PBS, they were centrifuged at 1,000 ×g for 5 min at 4 ℃ and resuspended in 10 µL of 1× binding buffer. Next, cells were stained with annexin V (5 µL) and PI (10 µL) for 5 min on ice. Subsequently, we used a flow cytometer (Quanteon; NovoCyte ACEA Biosciences, San Diego, CA, USA) to determine the relative fluorescence intensity. Apoptotic cells were then analyzed using NovoExpress 1.4.1 (Agilent Technologies, Santa Clara, CA, USA).
Western blotting
In this experiment, we firstly collected lysates (total-cell protein) using RIPA lysis buffer. Then, we transferred whole-cell protein lysates to PVDF membranes after electrophoresis through a sodium dodecyl sulfate (SDS) polyacrylamide gel. Following blocking with 5% (w/v) skim milk in Tris-buffered saline and Triton 100 (TBST) for 1 h, the PVDF membranes were incubated overnight at 4 ℃ using specific primary antibodies. The following day, we washed PVDF membranes thrice in TBST. After washing, the membranes were incubated with a secondary antibody, conjugated to horseradish peroxidase in 5% TBST for 1 h at room temperature, then washed again with TBST. Bands were quantified using Image Lab 5.2.1 software (Bio-Rad Laboratories, Hercules, CA, USA).
ELISAs
ELISA kits for IL-8 and IL-6 were used to measure the levels of proinflammatory cytokines in cell-free supernatants. Briefly, supernatants were collected after centrifugation at 2,000 ×g for 10 min at 4 ℃. Each sample was loaded onto a 96-well plate (50 µL/well). Then, 50 µL/well of an “antibody cocktail” (mixture of capture antibodies and detector antibodies prepared immediately before experimentation) was added to each well, followed by incubation for 60 min at room temperature on a plate shaker set to 400 rpm. Then, 100 µL of 3,3’,5,5’-tetramethylbenzidine (TMB) Development Solution was added to each well, followed by incubation for 10 min in the dark on a plate shaker set to 400 rpm. Subsequently, stop solution (100 µL) was added, and the OD at 450 nm was measured immediately by a microplate reader (SpectraMax M2; Molecular Devices). Mouse ELISA kits for IgE, TSLP, IL-4, and IL-6 were used according to the manufacturer’s instructions.
Animals
A total of 12 male and 12 female specific pathogen-free (SPF) NC/Nga mice (weighing 18–20 g; aged 6 to 8 weeks) were obtained from Riken BioResource Center (Tsukuba, Japan). Before experimentation, mice were allowed to acclimatize to laboratory conditions for 1 week. Animal experimental procedures were conducted following protocols approved by the Laboratory Animal Research Committee of Guangdong Provincial Hospital of Chinese Medicine (No. 2020081), in compliance with the Animal Welfare Policy of Guangdong Provincial Hospital of Chinese Medicine of Guangzhou University of Chinese Medicine.
We housed mice in a condition-controlled room where the temperature was 22±3 ℃ and the humidity was 55%±5%. Under a 12-h light-dark cycle, mice were fed autoclaved chow and filtered tap water. Throughout the experiment, the same experimenter raised all animals in the same location at the same time. The sample size was determined from a similar experiment reported in the literature (40,41). Prior to the study, a protocol was prepared without registration. In the experiment, any symptoms caused by DNCB unrelated to the disease model were recorded as adverse events, which would terminate the experiment, and the mice were immediately euthanized. Animals were excluded if they died prematurely, preventing sample collection. After the NC/Nga mice were numbered by one researcher, another researcher took the numbers from a sealed, opaque envelope and randomly divided them into 4 groups of 6: control (vehicle group without DNCB treatment), model (with DNCB treatment), ISO (oral administration of ISO plus DNCB sensitization), and DEX (oral application of DEX plus DNCB treatment). Random numbers were generated using the standard = RAND () function in Microsoft Excel (42).
Animal experiments were performed under 2,2,2-tribromoethanol (Avertin, Sigma-Aldrich, USA) anesthesia. In order to make a stock solution, we dissolved 10 g of 2,2,2-tribromoethanol in 10 mL of tertamyl alcohol. Using a dilution of 1 ml of stock solution with 39 mL of 0.9% NaCl, a final working solution was prepared. The working solution was injected intraperitoneally (i.p.; 12–15 µL/g of body weight) into each mouse as part of the anesthesia process. On day 26, various tissue and blood specimens of mice were collected immediately after 2,2,2-tribromoethanol anesthesia. No animals were excluded from this study. Researchers collected and analyzed data in no specific order using blinded mice and samples.
Induction of AD-like inflammation in the back skin of mice
As previously reported, DNCB induces skin inflammation similar to AD in NC/Nga mice (30). In brief, the dorsal skin area (6 cm2) of mice was shaved with an electronic clipper, and any remaining hair was cleared using hair-removal cream (Nair™; Church and Dwight, Ewing, NJ, USA) 1 day before DNCB treatment.
Then, the shaved dorsal skin was sensitized by topical application of 1% DNCB (200 µL) in a 3:1 acetone/olive oil (w/v) mixture on days 1 and 5. In addition, aliquots of a DNCB working solution (20 µL) were applied to each side of the ears on day1 and 5. Then, lesioned mice were rechallenged with 0.5% DNCB 3 times per week.
For in vivo experiments, ISO (2.5 mg/mL) was dissolved in sterile PBS and administered (50 mg/kg/day, p.o.) for a second sensitization, which was determined from a similar experiment reported in the literature (43). Simultaneously, mice in the DEX group received DEX (1 mg/kg, p.o., once-daily). Control-group mice were administered with vehicle.
Hematoxylin and eosin (HE) and toluidine blue (TB) staining
On anesthesia day, we collected the dorsal skin from each mouse. After fixation in cold 4% paraformaldehyde for 24 h, skin tissues were embedded in paraffin. Paraffin-embedded skin tissues were then cut into 4 mm-thick sections. Afterwards, we mounted sectioned tissues on a silane-coated glass slide and stained them with HE or TB. In subsequent sections, a computer-aided image-analysis program (Image-Pro Plus 6.0; Media Cybernetics, Rockville, USA) was used to calculate the number of infiltrating inflammatory cells.
Clinical characteristics and ear thickness of DNCB-induced AD-like model mice
Deterioration of the back skin of mice due to AD was evaluated by the Merkmal of symptom score once weekly. This is based on 4 main clinical characteristics of AD (edema, erythema or hemorrhage, scaling or dryness, and excoriation or erosion) as described by Sone and colleagues (44). Clinical scoring was based on the following classification: 0, no lesions; 1, mild lesions; 2, moderate lesions; and 3, severe lesions. A high dermatitis score indicates more severe conditions than a low score, and a dermatitis score can range from 0 to 12. The ear thickness of mice was measured by an electronic micrometer (Guilin Guanglu Measuring Instrument Co., Ltd., Guangxi, China) every week.
Statistical analysis
Three parallel tests were conducted to obtain each measurement. Data were presented as mean ± standard deviation (SD) for all experiments. GraphPad Prism 8 (GraphPad La Jolla, USA) was used for statistical analysis. In our study, we performed Student’s t-test or one-way analysis of variance (one-way ANOVA), followed by Dunnett’s test. A P value of 0.05 was deemed significant. The animal experiment was performed only once.
Results
Effects of ISO on the viability of the mutated mast cell lines HMC1.1 and HMC1.2
Local infiltration and activation of mast cells in skin are important features of the immune response, especially in AD. Mutated human mast cells (HMC1.1 cells and HMC1.2 cells) with spontaneous proliferation and activation are regarded as good models for AD research. We aimed to ascertain whether ISO can be employed to treat AD.
Firstly, we examined the effects of ISO on the growth of mutated human mast cells. The cytotoxic effects of ISO were evaluated after treatment with ISO (0–100 µM) for 24 h using the CCK8 assay. The half-maximal inhibitory concentrations for ISO in HMC1.1 cells and HMC1.2 cells were 13.04 µM and 303 µM at 24 h, respectively. Hence, ISO had a minimal effect on the viability of HMC1.2 cells at concentrations of 5, 10, 25, 50, and 100 µM (Figure 2A-2C). In particular, the viability of HMC1.1 cells exposed to ISO was significantly lower compared with that of control cells at low concentrations in a dose-dependent manner (Figure 2D-2F). Therefore, ISO concentrations of 5, 10, 25, 50, and 100 µM were considered suitable for further study in HMC1.1 cells.

ISO increases the apoptosis of HMC1.1 Cells
We aimed to explore whether ISO induces the apoptosis of HMC1.1 cells. We seeded cells (3×106 cells/well) into each well of 6-well plates. Then, we treated cells with the indicated concentration of ISO for 2 h before they were stimulated with PMA (0.05 µM) and calcium ionophore A23187 (1 µM). Apoptosis induction was assessed using flow cytometry after 24 h of exposure to different concentrations of ISO. ISO treatment could induce apoptosis in a concentration-dependent manner (Figure 3).

To verify our flow cytometry results, we investigated caspase cleavage produced by the cells after 24 h of exposure to different concentrations of ISO. As a result of ISO treatment, BAX and caspase-3 cleavage levels increased dose dependently (Figure 4A,4B). Overall, data matched the results obtained by flow cytometry.

ISO inhibits CD177/JAK/STAT signaling in a phosphatase-dependent manner in HMC1.1 cells
Next, we explored the molecular effects of ISO on the proteins involved in intracellular signaling. JAK2 and its downstream factors STAT1, STAT3, and STAT5 (JAK2-STAT signaling pathway) were triggered by stimulation with PMA and calcium ionophore A23187. This activation of the JAK/STAT signaling pathway can stimulate proinflammatory factors and promote the proliferation of mutated mast cells. Proinflammatory factors can, in turn, trigger multiple intracellular signal transduction pathways, such as the JAK/STAT pathway, which is a proinflammatory and apoptotic pathway.
Western blotting revealed that ISO (5, 10, 25 µM) could inhibit phosphorylation of CD177, JAK2, STAT1, STAT3, and STAT5 (Figure 4C,4D). As a result of ISO treatment, a dose-dependent reduction of tyrosine phosphorylation was observed for CD177, STAT3, JAK2, STAT1, and STAT5. A reduction of JAK2 phosphorylation can compromise its effectors (STAT1, STAT3, STAT5).
ISO downregulates expression of proinflammatory factors in HMC1.1 cells
ISO was tested for its anti-inflammatory effects by measuring IL-6 and IL-8 production in HMC1.1 cell supernatants. When exposed to PMA and calcium ionophore A23187, HMC1.1 cells consistently secreted large amounts of IL-6 protein and IL-8 protein. In PMA- and calcium ionophore A23187-stimulated HMC1.1 cells, ISO (5–25 mM) increased the expression of IL-6 protein and IL-8 protein (Figure 5A,5B).

Effect of ISO on DNCB-induced infiltration of immune cells
A clinical feature of AD is the impaired barrier function of the epidermal layer of skin. This feature contributes to increased infiltration of immune cells (especially mast cells and eosinophils) (13). Mice treated with ISO had fewer mast cells (Figure 6A,6B) and eosinophils (Figure 6C,6D) on their back skin than mice in the control group.

ISO ameliorates DNCB-induced AD-like symptoms
The in vivo experimental regimen is shown in Figure 7A. The lymph node weight decreased significantly under ISO treatment (Figure 7B). DNCB application induced thymic atrophy (decreased thymic weight index, Figure 7C), and ISO administration could alter these changes and spleen swelling (increased spleen weight index, Figure 7D).

When mice were treated with DNCB locally, the ears (Figure 7E) were significantly thicker than those in the control group. The ISO group and DEX group had a reduced ear thickness more than mice in the model group (Figure 7E).
ISO downregulates the expression of proinflammatory cytokines in serum in AD-like model mice
Molecular features of AD patients include increased expression of IL-4, IgE, TSLP, and IL-6. Downregulated expression of these cytokines was documented in ISO -treated mice (Figure 8A-8D).

Effect of ISO on DNCB-induced thickness of the epidermis and dermis
Repeated application of ISO or DEX was undertaken in NC/Nga mice for 3 weeks. DNCB-induced AD lesions (edema, hemorrhage, excoriation, scaling) were diminished by treatment with ISO or DEX (Figure 9A).

The dermis (Figure 9B) and epidermis (Figure 9C,9D) of DNCB-treated mice were thicker than those of control mice. In the DNCB-treated group, the dermis (Figure 9B) and epidermis (Figure 9C,9D) were thicker than those of the normal control group. Repair of the epidermis and dermis in the positive control group and ISO group resembled that of normal skin (Figure 9A-9D).
Discussion
Our study identified the regulatory mechanisms of ISO ‘s inhibitory effects on AD mice treated with DNCB and HMC1.1 mast cells stimulated by PMA/A23187. We found that ISO significantly suppressed the severity of the AD model both in vitro and in vivo, which suggested that it may exhibit protective properties against AD.
Early studies indicated that HDM (45), DNCB (46), ovalbumin (47), and calcipotriol (48) cause exacerbation of AD. DNCB is often used to induce AD in mouse models of AD, as the results are highly reproducible and repeatable (49-51).
As a representative in vivo model that mimics human AD symptoms, the NC/Nga mouse model is widely used to investigate drugs or natural candidates for AD treatment (52-54). Here, we treated NC/Nga mice with DNCB as an AD model based on the modified method described by our previous work (30).
The cause of AD can be attributed to a variety of factors. A crucial role for mast cells and their local infiltration in AD is to facilitate immune and inflammatory responses (13). According to several studies, mast cells (the primary source of histamine in skin) are critical to AD pathogenesis (55,56). It is important to identify agents that target these cells in order to develop new strategies for treating AD.
A potential way of carrying out this strategy is through regulation of the number and activation of mast cells, thereby affecting the initiation, perpetuation, and severity of inflammation. Therefore, we first investigated the therapeutic potential of ISO in mutated human mast cells (HMC1.1 and HMC1.2).
It was surprising to find that ISO can decrease the proliferation of mutated mast cells and induce their apoptosis, especially for HMC1.1 cells with the C-KIT V560G mutation, in a dose-dependent manner. Hence, we hypothesized that ISO’s immunomodulatory and anti-inflammatory effects might be focused on mast cells in AD. A23187 and PMA can induce cytokine and chemokine changes in human mast cells, while downregulating them can protect against inflammatory skin diseases (57,58). Then, we observed the anti-inflammatory effects of ISO. Based on the data, A23187/PMA induced upregulation of inflammatory cytokines IL-6 and IL-8 in HMC1.1 cells, and ISO reduced the levels of these inflammatory cytokines, which supported our speculation.
Reports have suggested that the JAK2/STAT signaling pathway has an essential role in cellular processes and immune functions (59). Serving as an upstream effector of JAK2/STAT signaling, CD177 plays a vital role in regulating cell proliferation (60). Furthermore, 3 members of the STAT protein family (STAT1, STAT3, STAT5) are integral transcription factors that translocate into the nucleus upon phosphorylation by receptor-associated kinases. Once activated by these receptors, phosphorylated STAT proteins can modulate nuclear gene expression, thereby having important roles in the cell growth and death of abnormal cells (61-63). Thus, our next objective was to determine whether ISO affects HMC1.1 cells and if any potential targets (CD177, JAK2, STAT1, STAT3, STAT5) exist. We demonstrated that ISO inhibits autonomous proliferation, apoptosis promotion, and the inflammatory response of HMC1.1 cells by blocking CD177/JAK2/STAT signaling. Besides, ISO increased the expression of caspase-3 cleavage and BAX in HMC1.1 cells, which strengthens the evidence that ISO induces the apoptosis of mast cells. Hence, improving apoptosis could be an additional anti-AD effect of ISO.
Furthermore, we examined the therapeutic effects of ISO against a mouse model of AD induced by DNCB, as well as its anti-inflammatory and immunomodulatory effects. Disruption of Th1/Th2 cytokine secretion is considered a pathological factor in AD (1). The cytokine IL-6 is produced by keratinocytes and was shown to play an essential role in inflammatory disorders such as psoriasis and AD (64). According to the study, IL-6 is expressed in response to a wide range of inflammatory stimuli (65). Researchers have demonstrated that human primary keratinocytes from AD patients have an intrinsically reduced capacity to produce IL-6 (66), and the levels of IL-6 in serum are markedly increased in AD patients (67). According to this study, ISO decreased the serum levels of IL-6 in AD-like mice, indicating that ISO may have anti-inflammatory properties. AD involves inflammation and atopic responses triggered by type 2 cytokines, such as IL-4 (1). We found that ISO significantly inhibited serum IL-4 levels, which are thought to be responsible for triggering AD’s type 2 immune response. Results from previous AD studies are in agreement with these results (68,69).
An increase in IgE levels along with immune cell infiltration are the hallmarks of AD-like skin inflammation mediated by activated Th2 cells (70,71). The role of mast cells in skin inflammation is well known. High levels of IgE have been shown to activate mast cells. An allergic reaction involves the release of histamine from mast cells due to the FcεRI IgE receptor on their surface (72). Consequently, methods that reduce mast cell counts and inhibit mast cell activation may be effective in alleviating AD symptoms. Our data revealed that the dorsal skin tissues of DNCB-sensitized mice had high levels of inflammatory cell infiltration (mast cells, eosinophils), while application of ISO can reduce these cells. Previous research supports that serum levels of epithelial cell-derived TSLP were significantly elevated in AD patients, which can initiate the Th2 and Th22 immune response (73) and activate neurons to induce itch (74). ISO reduced serum IgE and TSLP levels in mice induced by DNCB to produce AD-like symptoms. We also noted a marked alleviation of skin lesion manifestation (edema, hemorrhage, excoriation, scaling) and ear thickness upon ISO administration in AD-like model mice induced by DNCB. Skin lesion recovery was associated with a decrease in mast cells and infiltrating eosinophils.
An inflammatory response to DNCB causes lymph nodes to enlarge. Using the lymph nodes and spleen morphology as a method of comparing ISO treatment results, we observed the lymph nodes and spleens of the AD-only and ISO-treated groups. By comparing the AD-only group to the control group, Figure 7B shows a drastic increase in lymph node weight due to AD induction. Compared to the AD group, ISO and DEX treatments decreased the weight of lymph nodes. We propose that the alleviation was achieved by the transdermal route, in which medicine penetrated the skin and transferred to the lymph nodes through lymphatic drainage, which controls antigen cells and lymphocytes. As a result of this, the adaptive immune system is also activated (75). Our study suggests that ISO treatment reduced enlargement of lymphatic-related organs, including lymph nodes, by minimizing immune overreaction.
Aside from causing skin lesions and lymph node enlargement, AD can also affect the spleen and thymus. As shown in Figure 7D, among AD-only mice, the spleen index increased significantly compared to the control group due to induced AD. Compared to the AD group, ISO and DEX treatment decreased the spleen index. As shown in Figure 7C, DEX treatment decreased the thymus index, while ISO application increased the thymus index. Compared to the normal control group, the DNCB-treated group tended to have a thicker dermis and epidermis. The epidermis and dermis of the DEX group and ISO group were repaired similarly to normal skin. These data are consistent with the reduction in infiltrated mast cells and eosinophils.
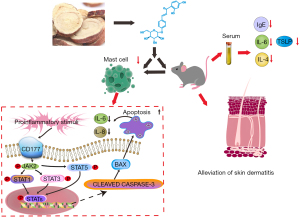
Overall, ISO, as demonstrated in the present study, exerts positive effects on AD-like lesions stimulated by DNCB. The results indicated that ISO inhibited proinflammatory chemokines and promoted cell apoptosis in PMA/A23187-treated HMC1.1 cells through inhibiting the CD177/JAK/STAT signaling pathway (Figure 10) in in vitro experiments.
There were some limitations to the present study. Firstly, our results confirmed the inhibitory effect of ISO on AD in vivo and in vitro, but due to the small number of animals, there were still some errors. In addition, there are also differences between nude mice and humans. The effectiveness and safety of ISO in alleviating AD are worthy of analysis in humans.
Conclusions
We demonstrated that ISO suppresses the proliferation, facilitates the apoptosis, and reduces the levels of proinflammatory cytokines in HMC1.1 cells by inhibiting the CD177/JAK/STAT signaling pathway. Furthermore, ISO treatment reduced immune cell infiltration (especially mast cells), ameliorated skin inflammation (alleviated skin lesions, reduced the thickness of the epidermis, dermis, and ear, and reduced the spleen index but increased the thymus index), and significantly reduced the expression of AD-related cytokines (IL-4, IgE, IL-6, and TSLP) in NC/Nga mice with AD induced by DNCB. Our study provides a foundation for the development of ISO-based agents based on the CD177/JAK2/STAT signaling pathway.
Acknowledgments
Funding: This work was funded by the National Natural Science Foundation of China Youth Fund (82004364); Second Affiliated Hospital of Guangzhou University of Chinese Medicine (SZ2021ZZ17); Guangdong Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Diseases (2018B030322012 and MB2020KF04); Guangdong-Hong Kong-Macau Joint Laboratory on Chinese Medicine and Immune Disease Research, Guangzhou University of Chinese Medicine (2020B1212030006); Key-Area Research and Development Program of Guangdong Province (2020B1111100010); National Administration of Traditional Chinese Medicine Inheritance and Innovation “Hundred and Ten Million” Talent Project (Qihuang project) Qihuang Scholar Project (Guo Zhong Yi Yao Ren Jiao Han [2018]: 284); and Chen Da-Chan Famous Medical Studio Project of Guangdong Provincial Hospital of Traditional Chinese Medicine (Y0096).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3989/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3989/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3989/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. Animal experimental procedures were conducted following protocols approved by the Laboratory Animal Research Committee of Guangdong Provincial Hospital of Chinese Medicine (No. 2020081), in compliance with the Animal Welfare Policy of Guangdong Provincial Hospital of Chinese Medicine of Guangzhou University of Chinese Medicine.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ständer S. Atopic Dermatitis. N Engl J Med 2021;384:1136-43. [Crossref] [PubMed]
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020;396:345-60. [Crossref] [PubMed]
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy 2018;73:1284-93. [Crossref] [PubMed]
- Pezzolo E, Naldi L. Epidemiology of major chronic inflammatory immune-related skin diseases in 2019. Expert Rev Clin Immunol 2020;16:155-66. [Crossref] [PubMed]
- Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol 2021;184:304-9. [Crossref] [PubMed]
- Ravnborg N, Ambikaibalan D, Agnihotri G, et al. Prevalence of asthma in patients with atopic dermatitis: A systematic review and meta-analysis. J Am Acad Dermatol 2021;84:471-8. [Crossref] [PubMed]
- Mukovozov IM, Morra DE, Giustini D, et al. Atopic dermatitis and bone health: a systematic review. J Eur Acad Dermatol Venereol 2021;35:615-28. [Crossref] [PubMed]
- Pan TL, Bai YM, Cheng CM, et al. Atopic dermatitis and dementia risk: A nationwide longitudinal study. Ann Allergy Asthma Immunol 2021;127:200-5. [Crossref] [PubMed]
- Sherman S, Kridin K, Bitan DT, et al. Hidradenitis suppurativa and atopic dermatitis: A 2-way association. J Am Acad Dermatol 2021;85:1473-9. [Crossref] [PubMed]
- Knudgaard MH, Andreasen TH, Ravnborg N, et al. Rhinitis prevalence and association with atopic dermatitis: A systematic review and meta-analysis. Ann Allergy Asthma Immunol 2021;127:49-56.e1. [Crossref] [PubMed]
- Richard MA, Sei JF, Philippe C, et al. Prevalence of comorbidities in atopic dermatitis and psoriasis in the French population. Ann Dermatol Venereol 2021;148:28-33. [Crossref] [PubMed]
- Thangam EB, Jemima EA, Singh H, et al. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front Immunol 2018;9:1873. [Crossref] [PubMed]
- Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol 2011;41:298-310. [Crossref] [PubMed]
- Hammad H, Lambrecht BN. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015;43:29-40. [Crossref] [PubMed]
- Chauvot de Beauchêne I, Allain A, Panel N, et al. Hotspot mutations in KIT receptor differentially modulate its allosterically coupled conformational dynamics: impact on activation and drug sensitivity. PLoS Comput Biol 2014;10:e1003749. [Crossref] [PubMed]
- Chan IJ, Kasprowicz S, Tharp MD. Distinct signalling pathways for mutated KIT(V560G) and KIT(D816V) in mastocytosis. Clin Exp Dermatol 2013;38:538-44. [Crossref] [PubMed]
- Frølunde AS, Thyssen JP, Deleuran M, et al. Appraisal of Proactive Topical Therapy in Atopic Dermatitis: Pros and Cons. Am J Clin Dermatol 2021;22:775-83. [Crossref] [PubMed]
- Gabros S, Nessel TA, Zito PM. Topical Corticosteroids. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2021.
- Siegels D, Heratizadeh A, Abraham S, et al. Systemic treatments in the management of atopic dermatitis: A systematic review and meta-analysis. Allergy 2021;76:1053-76. [Crossref] [PubMed]
- Coondoo A, Phiske M, Verma S, et al. Side-effects of topical steroids: A long overdue revisit. Indian Dermatol Online J 2014;5:416-25. [Crossref] [PubMed]
- Zheng T, Fan M, Wei Y, et al. Huangbai Liniment Ameliorates Skin Inflammation in Atopic Dermatitis. Front Pharmacol 2021;12:726035. [Crossref] [PubMed]
- Yan F, Li F, Liu J, et al. The formulae and biologically active ingredients of Chinese herbal medicines for the treatment of atopic dermatitis. Biomed Pharmacother 2020;127:110142. [Crossref] [PubMed]
- Yu Z, Deng T, Wang P, et al. Ameliorative effects of total coumarins from the fructus of Cnidium monnieri (L.) Cuss. on 2,4-dinitrochlorobenzene-induced atopic dermatitis in rats. Phytother Res 2021;35:3310-24. [Crossref] [PubMed]
- Xu Y, Chen S, Zhang L, et al. The Anti-Inflammatory and Anti-Pruritus Mechanisms of Huanglian Jiedu Decoction in the Treatment of Atopic Dermatitis. Front Pharmacol 2021;12:735295. [Crossref] [PubMed]
- Wang L, Xian YF, Loo SKF, et al. Baicalin ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice through modulating skin barrier function, gut microbiota and JAK/STAT pathway. Bioorg Chem 2022;119:105538. [Crossref] [PubMed]
- Wang Z, Wang ZZ, Geliebter J, et al. Traditional Chinese medicine for food allergy and eczema. Ann Allergy Asthma Immunol 2021;126:639-54. [Crossref] [PubMed]
- Gu SX, Mo X, Zhang AL, et al. A Chinese herbal medicine preparation (Pei Tu Qing Xin) for children with moderate-to-severe atopic eczema: a pilot randomized controlled trial. Br J Dermatol 2018;179:1404-5. [Crossref] [PubMed]
- Liu J, Mo X, Wu D, et al. Efficacy of a Chinese herbal medicine for the treatment of atopic dermatitis: a randomised controlled study. Complement Ther Med 2015;23:644-51. [Crossref] [PubMed]
- Gu SX, Zhang AL, Coyle ME, et al. Chinese herbal medicine granules (PTQX) for children with moderate to severe atopic eczema: study protocol for a randomised controlled trial. Trials 2015;16:294. [Crossref] [PubMed]
- Yan F, Zhang J, Li X, et al. Therapeutic Effects of Chinese Herbal Formula (PTQX) on NC/Nga Mice with Atopic Dermatitis-Like Skin Lesions. Evid Based Complement Alternat Med 2019;2019:8359252. [Crossref] [PubMed]
- Yu C, Zhang Y, Gao KX, et al. Serotonergically dependent antihyperalgesic and antiallodynic effects of isoliquiritin in a mouse model of neuropathic pain. Eur J Pharmacol 2020;881:173184. [Crossref] [PubMed]
- Luo J, Li Z, Wang J, et al. Antifungal Activity of Isoliquiritin and Its Inhibitory Effect against Peronophythora litchi Chen through a Membrane Damage Mechanism. Molecules 2016;21:237. [Crossref] [PubMed]
- Li Y, Song W, Tong Y, et al. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J Neuroinflammation 2021;18:1. [Crossref] [PubMed]
- Upadhyay S, Mantha AK, Dhiman M. Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes. J Ethnopharmacol 2020;258:112690. [Crossref] [PubMed]
- Liu Y, Xu X, Xu R, et al. Renoprotective Effects Of Isoliquiritin Against Cationic Bovine Serum Albumin-Induced Membranous Glomerulonephritis In Experimental Rat Model Through Its Anti-Oxidative And Anti-Inflammatory Properties. Drug Des Devel Ther 2019;13:3735-51. [Crossref] [PubMed]
- Liu YY, Wu JQ, Fan RY, et al. Isoliquiritin promote angiogenesis by recruiting macrophages to improve the healing of zebrafish wounds. Fish Shellfish Immunol 2020;100:238-45. [Crossref] [PubMed]
- Zhang XH, Zhou CC, Li CY, et al. Isoliquiritin exert protective effect on telencephalon infarction injury by regulating multi-pathways in zebrafish model of ischemic stroke. Phytomedicine 2021;83:153469. [Crossref] [PubMed]
- Kim JH, Kim W. Alleviation effects of Rubus coreanus Miquel root extract on skin symptoms and inflammation in chronic atopic dermatitis. Food Funct 2022;13:2823-31. [Crossref] [PubMed]
- Hong SM, Kang MC, Jin M, et al. Fermented blueberry and black rice containing Lactobacillus plantarum MG4221: a novel functional food for particulate matter (PM2.5)/dinitrochlorobenzene (DNCB)-induced atopic dermatitis. Food Funct 2021;12:3611-23. [Crossref] [PubMed]
- Park G, Moon BC, Choi G, et al. Cera Flava Alleviates Atopic Dermatitis by Activating Skin Barrier Function via Immune Regulation. Int J Mol Sci 2021;22:7531. [Crossref] [PubMed]
- Song HK, Park SH, Kim HJ, et al. Alpinia officinarum water extract inhibits the atopic dermatitis-like responses in NC/Nga mice by regulation of inflammatory chemokine production. Biomed Pharmacother 2021;144:112322. [Crossref] [PubMed]
- Yao PL, Morales JL, Zhu B, et al. Activation of peroxisome proliferator-activated receptor-β/δ (PPAR-β/δ) inhibits human breast cancer cell line tumorigenicity. Mol Cancer Ther 2014;13:1008-17. [Crossref] [PubMed]
- Shin YW, Bae EA, Lee B, et al. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Med 2007;73:257-61. [Crossref] [PubMed]
- Reigada I, Moliner C, Valero MS, et al. Antioxidant and Antiaging Effects of Licorice on the Caenorhabditis elegans Model. J Med Food 2020;23:72-8. [Crossref] [PubMed]
- Braz JM, Dembo T, Charruyer A, et al. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc Natl Acad Sci U S A 2021;118:e2021386118. [Crossref] [PubMed]
- Kim SY, Han SD, Kim M, et al. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants (Basel) 2021;10:1941. [Crossref] [PubMed]
- Leyva-Castillo JM, Sun L, Wu SY, et al. Single-cell transcriptome profile of mouse skin undergoing antigen-driven allergic inflammation recapitulates findings in atopic dermatitis skin lesions. J Allergy Clin Immunol 2022;150:373-84. [Crossref] [PubMed]
- Pellefigues C, Naidoo K, Mehta P, et al. Basophils promote barrier dysfunction and resolution in the atopic skin. J Allergy Clin Immunol 2021;148:799-812.e10. [Crossref] [PubMed]
- Kim YE, Choi SW, Kim MK, et al. Therapeutic Hydrogel Patch to Treat Atopic Dermatitis by Regulating Oxidative Stress. Nano Lett 2022;22:2038-47. [Crossref] [PubMed]
- Yang L, Fu J, Han X, et al. Hsa_circ_0004287 inhibits macrophage-mediated inflammation in an N6-methyladenosine-dependent manner in atopic dermatitis and psoriasis. J Allergy Clin Immunol 2022;149:2021-33. [Crossref] [PubMed]
- Yeo H, Ahn SS, Jung E, et al. Transcription Factor EGR1 Regulates the Expression of the Clock Gene PER2 under IL-4 Stimulation in Human Keratinocytes. J Invest Dermatol 2022; Epub ahead of print. [Crossref] [PubMed]
- Kanemaru K, Noguchi E, Tahara-Hanaoka S, et al. Clec10a regulates mite-induced dermatitis. Sci Immunol 2019;4:eaax6908. [Crossref] [PubMed]
- Park G, Lee SH, Oh DS, et al. Melatonin inhibits neuronal dysfunction-associated with neuroinflammation by atopic psychological stress in NC/Nga atopic-like mouse models. J Pineal Res 2017;63:e12420. [Crossref] [PubMed]
- Chiu YH, Wu YW, Hung JI, et al. Epigallocatechin gallate/L-ascorbic acid-loaded poly-γ-glutamate microneedles with antioxidant, anti-inflammatory, and immunomodulatory effects for the treatment of atopic dermatitis. Acta Biomater 2021;130:223-33. [Crossref] [PubMed]
- Leyva-Castillo JM, Galand C, Kam C, et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity 2019;50:1262-1275.e4. [Crossref] [PubMed]
- Serhan N, Basso L, Sibilano R, et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat Immunol 2019;20:1435-43. [Crossref] [PubMed]
- Chiu KM, Hung YL, Wang SJ, et al. Anti-Allergic and Anti-Inflammatory Effects of Neferine on RBL-2H3 Cells. Int J Mol Sci 2021;22:10994. [Crossref] [PubMed]
- Han NR, Ko SG, Moon PD, et al. Chloroquine attenuates thymic stromal lymphopoietin production via suppressing caspase-1 signaling in mast cells. Biomed Pharmacother 2021;141:111835. [Crossref] [PubMed]
- Zhang W, Zhao H, Chen J, et al. Mining database for the expression and gene regulation network of JAK2 in skin cutaneous melanoma. Life Sci 2020;253:117600. [Crossref] [PubMed]
- Stroncek DF, Caruccio L, Bettinotti M. CD177: A member of the Ly-6 gene superfamily involved with neutrophil proliferation and polycythemia vera. J Transl Med 2004;2:8. [Crossref] [PubMed]
- Mohassab AM, Hassan HA, Abdelhamid D, et al. STAT3 transcription factor as target for anti-cancer therapy. Pharmacol Rep 2020;72:1101-24. [Crossref] [PubMed]
- Shao S, Tsoi LC, Sarkar MK, et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci Transl Med 2019;11:eaav7561. [Crossref] [PubMed]
- Subramaniam D, Angulo P, Ponnurangam S, et al. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis 2020;11:149. [Crossref] [PubMed]
- Razali NA, Nazarudin NA, Lai KS, et al. Curcumin derivative, 2,6-bis(2-fluorobenzylidene)cyclohexanone (MS65) inhibits interleukin-6 production through suppression of NF-κB and MAPK pathways in histamine-induced human keratinocytes cell (HaCaT). BMC Complement Altern Med 2018;18:217. [Crossref] [PubMed]
- Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295. [Crossref] [PubMed]
- Niebuhr M, Heratizadeh A, Wichmann K, et al. Intrinsic alterations of pro-inflammatory mediators in unstimulated and TLR-2 stimulated keratinocytes from atopic dermatitis patients. Exp Dermatol 2011;20:468-72. [Crossref] [PubMed]
- Yeşilova Y, Çalka Ö, Akdeniz N, et al. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol 2012;24:189-93. [Crossref] [PubMed]
- Park CH, Min SY, Yu HW, et al. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int J Mol Sci 2020;21:4620. [Crossref] [PubMed]
- Lee Y, Choi HK, N'deh KPU, et al. Inhibitory Effect of Centella asiatica Extract on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Nutrients 2020;12:411. [Crossref] [PubMed]
- Furue M, Chiba T, Tsuji G, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int 2017;66:398-403. [Crossref] [PubMed]
- Werfel T, Allam JP, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol 2016;138:336-49. [Crossref] [PubMed]
- Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc 2019;40:84-92. [Crossref] [PubMed]
- Nygaard U, Hvid M, Johansen C, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol 2016;30:1930-8. [Crossref] [PubMed]
- Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155:285-95. [Crossref] [PubMed]
- Palmer BC, DeLouise LA. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. Molecules 2016;21:1719. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)