
The correlation factors and prognostic significance of coagulation disorders after chimeric antigen receptor T cell therapy in hematological malignancies: a cohort study
Introduction
Chimeric antigen receptor (CAR)-T cells have shown promising efficacy for the treatment of relapsed/refractory (r/r) hematologic malignancies, including acute lymphoblastic leukemia (ALL) (1,2), non-Hodgkin lymphoma (NHL) (3-6), and multiple myeloma (MM) (7,8). Consequently, 5 CAR-T products have been approved by the Food and Drug Administration (FDA) (2,3,5,6,8), which paved the way for the application of CAR-T treatment to a greater number of patients. However, CAR-T treatment-associated toxicities, which may be severe and life-threatening, cannot be disregarded. Thus far, most studies have focused on CAR-T-related cytokine release syndrome (CRS) and neurotoxicity (9-11).
Coagulation disorder is another common adverse reaction of CAR-T treatment. It has been reported that more than 50% of patients with hematological malignancies suffer from a coagulation disorder after CAR-T treatment (12,13), with some patients progressing to disseminated intravascular coagulation (DIC) (7–28%) (12,13) and pulmonary embolism (PE) (2–8%) (14), which seriously endangers the life of patients.
At present, there are few studies on the mechanisms and influencing factors of coagulopathy in CAR-T therapy. Wang et al. (12) and Shao et al. (15) found that the severity of coagulation disorders was positively correlated with CRS grade during CAR-T therapy. We know that most chemotherapeutic agents produce coagulopathy through induction of endothelial injury and decrease of coagulation factors synthesis in the liver in cancer patients (16). However, the association of coagulation disorders with endothelial and liver injury in CAR-T therapy has not been investigated yet. In addition, the prognostic significance of CAR-T-related coagulation disorders are not fully known.
Here, we explored the possible correlation factors and prognostic significance of coagulation disorders after CAR-T cell infusion in patients with r/r hematological malignancies. This study may provide new targets for the prevention of CAR-T-related coagulation disorders and new predictor for the prognosis of CAR-T therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3814/rc).
Methods
Patients
In this retrospective cohort study, patients with r/r hematological malignancies who were treated with CAR-T cells between April 2017 and February 2022 at The First Affiliated Hospital of Wenzhou Medical University were eligible. The following exclusion criteria were used: (I) patients with coagulation disorders and abnormal liver function before CAR-T cell infusion, (II) patients treated with anticoagulant drugs, and (III) patients who had clear signs of infection or other disorders known to cause coagulation disorders. The follow-up ended in April 2022. The number of cases in our hospital during the study period determined the sample size. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University (No. 2016-220 and 2017-159) and informed consent was taken from all the patients or the patients’ guardians.
CAR-T cell manufacture and infusion
In our study, CAR-T cell infusion was set on day 0. On approximately day-11, peripheral blood mononuclear cells were obtained from patients by leukapheresis, and CD3+ T cells were sorted for CAR-T cell preparation. The CAR included an anti-CD19 or B-cell maturation antigen (BCMA) single-chain variable fragment, 4-1BB or CD28 co-stimulatory domain, and CD3ζ T cell activation domain (Hrain Biotechnology and CARsgen Therapeutic, Shanghai, China). Prepared CAR-T cells were stored in liquid nitrogen until the day of infusion. Before infusion, all patients received lymphodepletion chemotherapy with fludarabine and cyclophosphamide (FC) regimen (fludarabine 25–30 mg/m2 on day-4 to -2, cyclophosphamide 500 mg/m2 on day-4 to -3).
CAR-T cell proliferation, CRS, and efficacy assessment
After CAR-T cell infusion, the patients’ physical condition was closely monitored for 14 days. On days 1, 3, 6, 9, 14, 21, and 28, peripheral blood was drawn for the assessment of circulating CAR-T cell and cytokine levels. From day 29 to 6 months post-infusion, CAR-T cell level and treatment response were assessed monthly. After 6 months, follow-up was performed at an interval of 3 months. CRS was diagnosed and graded according to the American Society for Transplantation and Cellular Therapy (ASTCT) consensus grading system (17). Grade ≥3 CRS was considered to be severe CRS. CAR-T cell levels were measured by quantitative polymerase chain reaction (qPCR), and treatment response was assessed according to the National Comprehensive Cancer Network (NCCN) guidelines by bone marrow blasts examination for ALL (18), the Lugano criteria by computed tomography (CT) and positron emission tomography (PET)/CT examination for NHL (19), and the International Myeloma Working Group (IMWG) criteria for MM (20). Response evaluation for MM included bone marrow plasma cells, serum paraprotein, immunofixation electrophoresis, and free immunoglobulin light chains. Progression-free survival (PFS) was defined as the time from CAR-T infusion to disease progression, death, or last follow-up.
Coagulation parameters, liver function, and von Willebrand Factor (VWF) monitoring
Coagulation disorders were defined as the abnormality in at least one coagulation parameters, including prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen, and D-dimer. These coagulation parameters were monitored closely within 1 month of CAR-T cell infusion. To further analyze the relationship of liver and endothelial cell damage with coagulation disorders in CAR-T treatment, we monitored liver function in all patients and measured the VWF level in 12 patients with coagulopathy. The above parameters of all patients were tested in the same laboratory.
Statistical analyses
Statistical analysis was performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Two-tailed P<0.05 was considered statistically significant. PFS was evaluated using Kaplan-Meier curves and compared using the log-rank test. Continuous variables and categorical variables were compared using Wilcoxon rank-sum test and Fisher’s exact test, respectively. The paired t-test was used to compare coagulation parameters and VWF level before and after CAR-T treatment. The correlation of 2 continuous variables was analyzed by Spearman correlation coefficient. To evaluate the independent predictors of PFS, variables with P<0.05 in the univariate analysis were included in the stepwise multivariate Cox regression analysis.
We analyzed whether PFS was related to sex, age, diagnosis, number of relapses, transplant history, tumor burden, CAR-T infusion dose, peak CAR-T cells, CAR-T expansion duration, and coagulation disorders. The cut-off values of CAR-T infusion dose, peak CAR-T cells, and CAR-T expansion duration were established based on the medians. PT, APTT, and TT prolongation were defined as exceeding the upper limit of normal by ≥3, ≥10, and ≥3 seconds, respectively. Fibrinogen concentration ≤1.5 g/L was defined as fibrinogen decrease, and D-dimer concentration ≥5 mg/L was defined as D-dimer increase.
Results
Patient characteristics
Between April 2017 and February 2022, 59 patients with r/r hematological malignancies were treated with CAR-T cells. A total of 56 patients [B-cell acute lymphoblastic leukemia (B-ALL), n=14; diffuse large B-cell lymphoma (DLBCL), n=15; MM, n=27] were included in our cohort study, and the remaining 3 patients were excluded. The median age was 52 years (range, 16–70 years). Comparisons of clinical characteristics among different coagulation disorder groups are listed in Table 1, and no significant differences were observed.
Table 1
| Characteristics | PT | APTT | TT | Fibrinogen | D-dimer | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (n=43) | Prolonged (n=13) | P | Normal (n=29) | Prolonged (n=27) | P | Normal (n=41) | Prolonged (n=15) | P | Normal (n=36) | Decreased (n=20) | P | Normal (n=34) | Elevated (n=22) | P | |||||
| Sex | 1 | 0.789 | 0.227 | 0.575 | 0.577 | ||||||||||||||
| Male | 26 [60] | 8 [61] | 17 [59] | 17 [63] | 27 [66] | 7 [47] | 23 [64] | 11 [55] | 22 [65] | 12 [54] | |||||||||
| Female | 17 [40] | 5 [39] | 12 [41] | 10 [37] | 14 [34] | 8 [53] | 13 [36] | 9 [45] | 12 [35] | 10 [45] | |||||||||
| Age | 1 | 0.795 | 1 | 1 | 0.414 | ||||||||||||||
| <50 years | 20 [47] | 6 [46] | 14 [48] | 12 [44] | 19 [46] | 7 [47] | 17 [47] | 9 [45] | 14 [41] | 12 [54] | |||||||||
| ≥50 years | 23 [53] | 7 [54] | 15 [52] | 15 [56] | 22 [54] | 8 [53] | 19 [53] | 11 [55] | 20 [59] | 10 [46] | |||||||||
| Diagnosis | 0.656 | 0.736 | 0.681 | 1 | 0.492 | ||||||||||||||
| MM | 20 [47] | 7 [54] | 15 [51] | 12 [44] | 20 [49] | 7 [47] | 17 [47] | 10 [50] | 16 [47] | 11 [50] | |||||||||
| B-ALL | 10 [23] | 4 [31] | 6 [21] | 8 [30] | 9 [22] | 5 [33] | 9 [25] | 5 [25] | 7 [21] | 7 [32] | |||||||||
| DLBCL | 13 [30] | 2 [15] | 8 [28] | 7 [26] | 12 [29] | 3 [20] | 10 [28] | 5 [25] | 11 [32] | 4 [18] | |||||||||
| Number of relapses | 1 | 0.43 | 0.768 | 0.785 | 0.581 | ||||||||||||||
| <2 | 18 [42] | 6 [46] | 14 [48] | 10 [37] | 17 [42] | 7 [47] | 16 [44] | 8 [40] | 16 [47] | 8 [36] | |||||||||
| ≥2 | 25 [58] | 7 [54] | 15 [52] | 17 [63] | 24 [58] | 8 [53] | 20 [56] | 12 [60] | 18 [53] | 14 [64] | |||||||||
| Transplant history | 0.514 | 1 | 0.524 | 0.552 | 0.573 | ||||||||||||||
| Yes | 28 [65] | 10 [77] | 20 [69] | 18 [67] | 29 [71] | 9 [60] | 23 [64] | 15 [75] | 22 [65] | 16 [73] | |||||||||
| No | 15 [35] | 3 [23] | 9 [31] | 9 [33] | 12 [29] | 6 [40] | 13 [36] | 5 [25] | 12 [35] | 6 [27] | |||||||||
| Tumor burden | 1 | 0.361 | 0.736 | 1 | 0.762 | ||||||||||||||
| Low | 11 [26] | 3 [23] | 9 [31] | 5 [19] | 11 [27] | 3 [20] | 9 [25] | 5 [25] | 8 [24] | 6 [27] | |||||||||
| High | 32 [74] | 10 [77] | 20 [69] | 22 [81] | 30 [73] | 12 [80] | 27 [75] | 15 [75] | 26 [76] | 16 [73] | |||||||||
| CAR-T infusion dose | 1 | 0.422 | 1 | 0.412 | 0.423 | ||||||||||||||
| <3×106/kg | 21 [49] | 6 [46] | 12 [41] | 15 [56] | 20 [49] | 7 [47] | 19 [53] | 8 [40] | 18 [53] | 9 [41] | |||||||||
| ≥3×106/kg | 22 [51] | 7 [54] | 17 [59] | 12 [44] | 21 [51] | 8 [53] | 17 [47] | 12 [60] | 16 [47] | 13 [59] | |||||||||
Numbers are presented as n [%]. PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; B-ALL, B-cell acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; MM, multiple myeloma; CAR-T, chimeric antigen receptor T cells.
Incidence of coagulation disorders
Within 1 month of CAR-T cell infusion, 59% of patients had at least 1 abnormal coagulation parameter. The medians of maximum PT, APTT, TT, and D-dimer were 15.4 seconds (13.5–26.4 seconds), 47.8 seconds (31.7–131.1 seconds), 17.5 seconds (14.5–27.1 seconds), and 3.04 mg/L (0.31–20.00 mg/L), respectively, and the median of minimum fibrinogen was 2.43 g/L (0.55–6.78 g/L). The incidence of prolonged PT, prolonged APTT, prolonged TT, decreased fibrinogen, and elevated D-dimer were 23%, 48%, 27%, 36%, and 39%, respectively. PT prolongation, APTT prolongation, TT prolongation, and D-dimer increase peaked at a median of 9 days (2–16 days), 6 days (1–16 days), 9 days (4–16 days), and 8 days (3–16 days), respectively, and fibrinogen decreased to its lowest value at a median of 12 days (7–21 days). There was a significant statistical difference for all values compared with their respective baseline level (Figure 1A-1E).
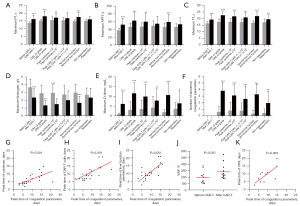
Association of coagulation disorders with CRS and CAR-T cell proliferation
The all-grade CRS rate and grade ≥3 CRS rate were 82% and 36%, respectively. We found that there were significant differences in maximum PT, APTT, TT, D-dimer, and minimum fibrinogen levels between severe and mild CRS patients (Figure 1A-1E). Moreover, patients with grade ≥3 CRS had more abnormal coagulation parameters than those with grade <3 CRS (Figure 1F). In our study, increased cytokines were mainly interleukin (IL)-6 and interferon (IFN)-γ; thus, the total peak level of cytokines was defined as the sum of the peak levels of IL-6 and IFN-γ. Further analysis showed that cytokine levels were correlated with the levels of maximum PT, APTT, TT, D-dimer, and minimum fibrinogen (Figure 1A-1E) and also with the number of abnormal coagulation parameters (Figure 1F). In addition, the peak time of coagulation parameters and cytokines was positively correlated (Figure 1G).
All CAR-T cells were derived from recipients, and the median infusion dose was 3×106/kg (1–28.2×106/kg). The median peak level, peak time, and expansion duration of CAR-T cells were 1.1×105 copies/µg genomic DNA (gDNA) (0.0009–18×105 copies/µg gDNA), 9 days (6–36 days), and 1.9 months (0–14.1 months), respectively. We found that the level of peak CAR-T cells was related to the levels of maximum PT, TT, D-dimer, minimum fibrinogen, and the number of abnormal coagulation parameters but not related to the level of maximum APTT (Figure 1A-1F). Moreover, the peak time of coagulation parameters was positively associated with CAR-T cells (Figure 1H).
Association of the coagulation disorders with liver damage and VWF increase
The incidence of abnormal liver function was 48% within 1 month of CAR-T cell infusion. The medians of maximum alanine aminotransferase and aspartate aminotransferase were 35 U/L (10–401 U/L) and 35 U/L (16–755 U/L), respectively. Further analysis showed that liver function was related to all coagulation parameters (Figure 1A-1F). Furthermore, the peak time of coagulation parameters and liver function parameters was positively correlated (Figure 1I). For endothelial cells, we found that the serum levels of VWF after CAR-T cell infusion was higher than baseline levels in the patients with coagulopathy (Figure 1J). Similarly, the peak time of coagulation parameters was positively associated with VWF (Figure 1K).
Association of the coagulation disorders with initial response rates and PFS
Fifty-three out of 56 patients (95%) survived for more than 1 month and were evaluated for response. The remaining 3 patients died of severe CRS or cerebral hemorrhage within 1 month of CART cell infusion. The overall response rate and complete remission rate without additional therapy after CAR-T infusion were 74% and 58%, respectively. Further analysis showed that initial response was associated with maximum TT and D-dimer but not associated with maximum PT, APTT and minimum fibrinogen levels (Figure 1A-1E). In addition, responders had more abnormal coagulation parameters than non-responders (Figure 1F).
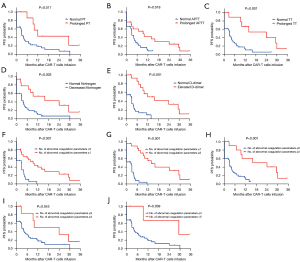
To avoid the impact of hematopoietic stem cell transplantation (HSCT) on the long-term efficacy of CART treatment, the 6 patients with CAR-T treatment bridging HSCT were excluded in the analysis of PFS. At a median follow-up of 26.8 months, the median PFS among the 47 evaluated patients was 4.1 months. To explore the relevant factors affecting PFS, we analyzed whether PFS was related to sex, age, diagnosis, number of relapses, transplant history, tumor burden, CAR-T infusion dose, peak CAR-T cells, CAR-T expansion duration, and coagulation disorders. In univariate analysis, we found that PFS was associated with peak CAR-T cells [hazard ratio (HR) =0.417, 95% confidence interval (CI): 0.208–0.837], CAR-T expansion duration (HR =0.475, 95% CI: 0.247–0.914), and also coagulation disorders, including PT prolongation (HR =0.363, 95% CI: 0.181–0.725), APTT prolongation (HR =0.516, 95% CI: 0.274–0.974), TT prolongation (HR =0.326, 95% CI: 0.172–0.619), fibrinogen decrease (HR =0.373, 95% CI: 0.198–0.701), and D-dimer increase (HR =0.304, 95% CI: 0.162–0.573) (Table 2). However, multivariable Cox regression analysis showed that only TT prolongation (HR =0.279, 95% CI: 0.099–0.782) and D-dimer increase (HR =0.218, 95% CI: 0.087–0.548) were independent predictors for PFS (Table 2). Patients with prolonged PT, prolonged APTT, prolonged TT, decreased fibrinogen, elevated D-dimer, or more abnormal coagulation parameters exhibited longer PFS (Figure 2).
Table 2
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Sex (male vs. female) | 0.755 | 0.402–1.418 | 0.357 | – | – | – | |
| Age (years) (<50 vs. ≥50) | 1.029 | 0.547–1.936 | 0.922 | – | – | – | |
| Diagnosis (B-ALL vs. DLBCL vs. MM) | |||||||
| B-ALL vs. DLBCL | 1.93 | 0.763–4.887 | 0.098 | – | – | – | |
| B-ALL vs. MM | 1.294 | 0.577–2.900 | 0.531 | – | – | – | |
| DLBCL vs. MM | 0.695 | 0.318–1.521 | 0.266 | – | – | – | |
| Times of relapse (<2 vs. ≥2) | 0.918 | 0.478–1.762 | 0.779 | – | – | – | |
| Transplant history (yes vs. no) | 1.284 | 0.649–2.541 | 0.412 | – | – | – | |
| Tumor burden (low vs. high) | 1.094 | 0.513–2.334 | 0.808 | – | – | – | |
| CAR-T infusion dose (×106/kg) (<3 vs. ≥3) | 1.29 | 0.688–2.419 | 0.394 | – | – | – | |
| Peak CAR-T cells (×105 copies/μg gDNA) (<1.1 vs. ≥1.1) | 0.417 | 0.208–0.837 | 0.001* | – | – | – | |
| CAR-T expansion duration (months) (<1.9 vs. ≥1.9) | 0.475 | 0.247–0.914 | 0.007* | – | – | – | |
| Coagulation disorders | |||||||
| PT (normal vs. prolonged) | 0.363 | 0.181–0.725 | 0.017* | – | – | – | |
| APTT (normal vs. prolonged) | 0.516 | 0.274–0.974 | 0.018* | – | – | – | |
| TT (normal vs. prolonged) | 0.326 | 0.172–0.619 | 0.001* | 0.279 | 0.099–0.782 | 0.015* | |
| Fibrinogen (normal vs. decreased) | 0.373 | 0.198–0.701 | 0.003* | – | – | – | |
| D-dimer (normal vs. elevated) | 0.304 | 0.162–0.573 | <0.001* | 0.218 | 0.087–0.548 | 0.001* | |
*, P<0.05. PFS, progression-free survival; B-ALL, B-cell acute lymphoblastic leukemia; DLBCL, diffuse large B-cell lymphoma; MM, multiple myeloma; CAR-T, chimeric antigen receptor T cells; gDNA, genomic DNA; PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time.
Discussion
Despite CAR-T cell therapy showing promising response rates, treatment-associated toxicities are still a major concern. Along with CRS and neurotoxicity, coagulation disorder is a common early complication of CAR-T treatment. In our study, the incidence of coagulation disorders was 59% within 1 month of CAR-T cell infusion in patients with r/r hematological malignancies. PT prolongation, APTT prolongation, TT prolongation, and D-dimer increase peaked at a median of 6–9 days, and fibrinogen decreased to its lowest value at a median of 12 days. The changes in coagulation parameters we observed were similar to previous reports (12,13). Therefore, it is important to be alert to the occurrence of coagulation disorders in patients treated with CAR-T cells, particularly in the second week after infusion.
To our knowledge, the mechanisms of CAR-T-related coagulation disorders remain unclear. Consistent with previous research (12,13,15), we found that coagulation disorders in patients with severe CRS were more significant. Our previous study found that CRS severity was associated with levels of cytokines and CAR-T cells (21), so we further investigated the relationship of cytokines and CAR-T cells with coagulation disorders. The results showed that abnormality of coagulation parameters was closely related to cytokines and CAR-T cells in both peak level and peak time.
We then considered the mechanism by which high levels of cytokines and CAR-T cells induced coagulation disorders. We know that many coagulation-related proteins are synthesized in the liver, and thus the liver plays an important role in the regulation of coagulation function (22). Our study showed that abnormality of coagulation parameters was also closely associated with liver function parameters in both peak level and peak time. Therefore, we speculated that high levels of cytokines and CAR-T cells may induce coagulation disorders, at least in part, by injuring the liver.
Besides the liver, we know that endothelial activation is also closely related to coagulation disorders (23). It has been demonstrated that high levels of cytokines (24,25) and CAR-T cells (26) can cause activation and damage of endothelial cells. In this study, we explored the presence of endothelial activation after CAR-T cell infusion in patients with coagulation disorders by detecting the serum levels of VWF, an important marker of endothelial activation (27). The results showed that the serum level of VWF after CAR-T cell infusion was higher than baseline levels in the patients with coagulopathy. Moreover, the peak time of coagulation parameters was positively associated with VWF. The above results suggested that CAR-T-related coagulation disorders may have been secondary to endothelial activation induced by high levels of cytokines and CAR-T cells.
In addition to the correlation factors of CAR-T-related coagulation disorders, we also explored the prognostic significance of coagulation disorders in patients treated with CAR-T cells. The results showed that coagulation disorders were associated with higher initial response rates and also longer PFS in CAR-T therapy, with TT prolongation and D-dimer increase independent predictors for PFS.
In conclusion, we propose the following possible mechanisms of CAR-T-related coagulation disorders: (I) high levels of cytokines cause liver damage and impair the production of coagulation-related proteins; (II) the liver damage caused by off-target effects of CAR-T cells also affects the production of coagulation-related proteins; (III) high levels of cytokines cause activation and damage of vascular endothelial cells, thereby activating coagulation pathway; (IV) CAR-T cells directly damage vascular endothelial cells, resulting in activation of coagulation. Further, our results suggested that coagulation disorders occurring within 1 month of CAR-T cell infusion could serve as a new predictor for prognosis in patients with r/r hematological malignancies. However, due to the limited number of cases in this study, our findings need to be further validated with larger, multicenter, and prospective studies.
Acknowledgments
Funding: This work was supported by the major project of Wenzhou Municipal Science and Technology Bureau (No. ZY2021013).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3814/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3814/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3814/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of The First Affiliated Hospital of Wenzhou Medical University (No. 2016-220 and 2017-159) and informed consent was taken from all the patients or the patients’ guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018;378:449-59. [Crossref] [PubMed]
- Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439-48. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45-56. [Crossref] [PubMed]
- Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med 2020;382:1331-42. [Crossref] [PubMed]
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396:839-52. [Crossref] [PubMed]
- Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma. Blood 2017;130:2594-602. [Crossref] [PubMed]
- Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med 2021;384:705-16. [Crossref] [PubMed]
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95. [Crossref] [PubMed]
- Li W, Wu L, Huang C, et al. Challenges and strategies of clinical application of CAR-T therapy in the treatment of tumors-a narrative review. Ann Transl Med 2020;8:1093. [Crossref] [PubMed]
- Greenbaum U, Kebriaei P, Srour SA, et al. Chimeric antigen receptor T-cell therapy toxicities. Br J Clin Pharmacol 2021;87:2414-24. [Crossref] [PubMed]
- Wang Y, Qi K, Cheng H, et al. Coagulation Disorders after Chimeric Antigen Receptor T Cell Therapy: Analysis of 100 Patients with Relapsed and Refractory Hematologic Malignancies. Biol Blood Marrow Transplant 2020;26:865-75. [Crossref] [PubMed]
- Jiang H, Liu L, Guo T, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol 2019;98:1721-32. [Crossref] [PubMed]
- Parks AL, Kambhampati S, Fakhri B, et al. Incidence, management and outcomes of arterial and venous thrombosis after chimeric antigen receptor modified T cells for B cell lymphoma and multiple myeloma. Leuk Lymphoma 2021;62:1003-6. [Crossref] [PubMed]
- Shao M, Yu Q, Teng X, et al. CRS-related coagulopathy in BCMA targeted CAR-T therapy: a retrospective analysis in a phase I/II clinical trial. Bone Marrow Transplant 2021;56:1642-50. [Crossref] [PubMed]
- Kvolik S, Jukic M, Matijevic M, et al. An overview of coagulation disorders in cancer patients. Surg Oncol 2010;19:e33-46. [Crossref] [PubMed]
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2019;25:625-38. [Crossref] [PubMed]
- Brown PA, Wieduwilt M, Logan A, et al. Guidelines Insights: Acute Lymphoblastic Leukemia, Version 1.2019. J Natl Compr Canc Netw 2019;17:414-23. [Crossref] [PubMed]
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059-68. [Crossref] [PubMed]
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328-e46. [Crossref] [PubMed]
- Dong R, Jiang S, Chen Y, et al. Prognostic Significance of Cytokine Release Syndrome in B Cell Hematological Malignancies Patients After Chimeric Antigen Receptor T Cell Therapy. J Interferon Cytokine Res 2021;41:469-76. [Crossref] [PubMed]
- Amitrano L, Guardascione MA, Brancaccio V, et al. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83-96. [Crossref] [PubMed]
- Neubauer K, Zieger B. Endothelial cells and coagulation. Cell Tissue Res 2022;387:391-8. [Crossref] [PubMed]
- Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017;130:2295-306. [Crossref] [PubMed]
- Gust J, Hay KA, Hanafi LA, et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov 2017;7:1404-19. [Crossref] [PubMed]
- Sun Y, Wang S, Zhao L, et al. IFN-gamma and TNF-alpha aggravate endothelial damage caused by CD123-targeted CAR T cell. Onco Targets Ther 2019;12:4907-25. [Crossref] [PubMed]
- Schwameis M, Schorgenhofer C, Assinger A, et al. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost 2015;113:708-18. [Crossref] [PubMed]


