
The efficacy of vonoprazan combined with different dose amoxicillin on eradication of Helicobacter pylori: an open, multicenter, randomized clinical study
Introduction
Chronic gastritis, peptic ulcer, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer are all intimately connected with Helicobacter pylori (H. pylori) infection. Worldwide, H. pylori-induced inflammation and damage account for around 75% of cases of stomach cancer (1,2). Gastric cancer and H. pylori infection are both quite common in China. In 2018, approximately half of estimated deaths from gastric cancer occurred in China (3,4). Currently, H. pylori infection is the most significant controllable risk factor in the prevention of gastric cancer, and its eradication may lower the chance of developing gastric cancer (5). At the moment, the triple regimen incorporating bismuth is advised as the first-line treatment option by China’s fifth consensus on the treatment of H. pylori infection (3). With this treatment, the eradication rate can increase to over 80%, but there some drawbacks remain, including a high rate of antibiotic resistance, the inadequacy of current therapies to address clinical needs, and the increased risk of drug side effects and poor medication compliance incurred by the use of multiple drugs and complex administration techniques. In order to obtain a satisfactory rate of eradication while avoiding antibiotic resistance, new strategies are required to facilitate minimal antibiotic administration and shorter treatment periods.
The use of vonoprazan (VPZ) has improved the eradication therapy in Japan. In the first clinical trial conducted in Japan, the eradication rate of VPZ combined with amoxicillin and clarithromycin triple therapy was 92.6%, and the control group containing PPI (such as lansoprazole) was 75.9%. The incidence of adverse reactions in VPZ group was 34.0%. The incidence of adverse drug reactions in lansoprazole group was 41.1% (for drug-related adverse reactions, respectively), and there was no significant difference between the two groups. The latest popular eradication standard regimen in Japan is triple regimen: VPZ 20 mg b.i.d, amoxicillin 750 mg b.i.d and clarithromycin 200 or 400 mg b.i.d, lasting for 7 days. And the eradication rate of clarithromycin-resistant patients reached 82%, which may suggest that the dual treatment of VPZ and amoxicillin can also reach about 82% eradication rate (6). Related studies in Japan have shown that the eradication rate of H. pylori with VPZ-based dual therapy is not inferior to that of a triple therapy containing clarithromycin (7,8). VPZ triple therapy has now become the recommended therapy in Japanese guidelines. After a number of randomized controlled trials, Japanese researchers have concluded that VPZ-based triple therapy has the best performance to eradicate H. pylori (9-13). However, it is known that the inhibitory effect of clarithromycin on P-glycoprotein and CYP3A4 can have different effects with related drugs, such as statins, cyclosporine, calcium channel blockers, carbamazepine, etc., it needs special attention to patients who use these drugs. Secondly, clarithromycin has the risk of prolonging QT interval, so there is a risk of sudden death due to arrhythmia when using clarithromycin (14). Therefore, considering the high drug resistance of clarithromycin and the above factors, we hope to achieve the eradication purpose without using clarithromycin in regimen.
In light of increasing antibiotic resistance, a proposed new first-line treatment for H. pylori is the combination of VPZ and amoxicillin. As far as we know, in China, there are few clinical trials on the treatment of H. pylori with VPZ, and there is a lack of large-sample, multi-center trials. There is no agreement on the dosage and frequency of amoxicillin administration in the VPZ dual treatment; this study probed the lack of standard dual therapies in China that combine VPZ and amoxicillin as well as the lack of a standard treatment plan for the local Chinese population. We present the following article in accordance with the CONSORT reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4133/rc).
Methods
Research design and ethics
An open, multicenter, randomized parallel controlled clinical research design was used in this investigation. Between December 2020 and May 2022, following screening for H. pylori infection, about 230 newly diagnosed H. pylori patients were enrolled in the outpatient clinics at Lanzhou University Second Hospital, No. 940 Hospital of Joint Service Support Force, and Gansu Provincial Hospital. The patients were then randomly assigned to 1 of 3 groups. The groups were distinguished by increasing the dose and frequency of amoxicillin in VPZ combined with amoxicillin and comparing it with clarithromycin triple therapy. The efficacy, safety, and adverse reactions of the high-dose amoxicillin triple therapy were comprehensively evaluated. The study was approved by the Medical Ethics Committee of Lanzhou University Second Hospital (No. 2020A-274). No. 940 Hospital of Joint Service Support Force and Gansu Provincial Hospital were informed and agreed with the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Participants
The participant inclusion criteria were as follows: (I) patients who were initially screened positive for H. pylori by 13C-urea breath test (UBT) or 14C-UBT and had not been treated; (II) patients aged 18–70 years, male or female; and (III) patients who signed the informed agreement for study and received treatment for eradicating H. pylori. The exclusion criteria were as follows: (I) allergy to any of the medications used in this medical trial; (II) use of proton pump inhibitors (PPIs), histamine H2 receptor antagonists, antibiotics, bismuth, or probiotics during the 4 weeks prior to beginning study treatment; (III) use of adrenocorticosteroids, non-steroidal anti-inflammatory drugs, and anticoagulants; (IV) alcoholism; and (V) maladies or health manifestations that may interact with the assessment of testing and treatment, including before screening processes. In order to determine whether patients were eligible, researchers evaluated them according to the inclusion and exclusion criteria, merged patient examination findings, baseline data, and clinical data, recorded baseline data, and assessed patients’ conditions.
Blinding and randomization
Using a computer-generated randomized sequence, patients were randomized to groups A, B, and C in the ratio of 1:1:1. The patients were asked to assent to the study and sign an informed consent form when it had been determined that they satisfied all inclusion criteria and none of the exclusion criteria. The patients were divided into groups based on a computer-generated randomized list, and each group received the proper course of treatment.
Both the researchers and the participants were aware of the treatment strategy. The urea breath testers were blinded to the patient classification and treatment strategy.
Clinical information collection
All participants were asked to provide a thorough medical history and the researchers collected demographic data, smoking history, history of alcohol consumption, family history, past medical history, and antibiotic medication history. If the patient had previously undergone gastroscopy, the gastroscopy and histological findings were recorded. At the same time, the digestive tract symptoms and their severity were recorded and evaluated. The Gastrointestinal Symptom Rating Scale (GSRS) score was used to evaluate the severity of symptoms: that is, the score range of each symptom was 0–3 points: 0 was asymptomatic; 1 was mildly symptomatic; 2 was symptom can be tolerated or relieved by drugs and 3 indicated extremely symptomatic and unbearable.
Grouping and treatment plans
After the screening activities, patients eligible for enrollment were randomly assigned to receive the following eradication regimens in a 1:1:1 ratio: (I) H-VA (high-dose amoxicillin combined with VPZ): VPZ 20 mg b.i.d + amoxicillin 750 mg q.i.d for 7 days; (II) L-VA (low-dose amoxicillin combined with VPZ): VPZ 20 mg b.i.d + amoxicillin 500 mg q.i.d for 7 days; (III) VAC (amoxicillin combined with VPZ and clarithromycin): VPZ 20 mg + amoxicillin 750 mg + clarithromycin 500 mg, twice a day for 7 days.
Results assessment
The primary endpoint is eradication rate, calculated by intention to treat (ITT) and per protocol (PP). We think that the acceptable eradication rate is higher than or equal to 80%.
Follow-up procedure: at the 1st and 4th week after the patients’ treatment, the doctor will contact the patient by telephone to record the medication situation and adverse reactions.
Compliance: ≥80% of the total dosage is considered as acceptable compliance.
Safety: evaluate the safety of different therapies by the incidence of adverse reactions.
At least 4 weeks after treatment, participants were reexamined with a urea breathalyzer test. The effectiveness of various regimens was assessed based on compliance, safety, and eradication rate.
Sample size estimation
The statistical software PASS (version 15.0.5; NCSS LLC, Kaysville, UT, USA) was used to determine the sample size for this investigation in accordance with the χ2 test for multiple proportions. Based on power (1 − β), alpha (significance level), effect size, and degrees of freedom, which were 0.80, 0.05, 0.22, and 2, respectively, at least 230 patients were required for this randomized clinical pilot research. The ultimate sample size for this research was determined at 230 patients using an assumed follow-up rate of loss of 15%.
Statistical analysis
The main results of each scheme were calculated by ITT and PP analysis. Differences in eradication rates were compared by the χ2 test. The quantitative data from a normal distribution or non-normal distribution were tested using the F test and H test, respectively. χ2 test or Fisher’s exact test were used to compare the categorical data between different schemes. All statistical analyses were carried out using SPSS 25.0 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
General information
Among the 273 H. pylori-positive individuals who were assessed for eligibility, 43 were disqualified because they did not match the requirements, declined to take part, or for other reasons. A total of 230 H. pylori-positive individuals were enrolled, and they were randomly assigned to undergo 7 days of L-VA, H-VA, or VAC treatment.
The details of participants in each group (age, gender, height, weight, smoking, alcohol consumption, and diagnosis) are shown in Table 1. There was no significant difference in baseline characteristics between groups (P>0.05).
Table 1
| Characteristics | H-VA | L-VA | VAC | P value |
|---|---|---|---|---|
| Age (years), median [P25, P75] | 43 [31, 52] | 40 [32, 50] | 39 [30, 50] | 0.823 |
| Gender (M/F) | 38/47 | 31/53 | 28/33 | 0.466 |
| Height (cm), median [P25, P75] | 165 [160, 172] | 163 [160, 171] | 165 [161, 171] | 0.366 |
| Weight (kg), median [P25, P75] | 59 [52, 67] | 60 [53, 70] | 60 [51, 65] | 0.722 |
| History of smoking | 16 | 12 | 14 | 0.405 |
| History of alcohol consumption | 30 | 26 | 16 | 0.985 |
| Family history | 12 | 14 | 6 | 0.501 |
H-VA: vonoprazan 20 mg b.i.d with amoxicillin 750 mg q.i.d for 7 days; L-VA: vonoprazan 20 mg b.i.d plus amoxicillin 500 mg q.i.d for 7 days; VAC: vonoprazan 20 mg b.i.d plus amoxicillin 750 mg plus clarithromycin 500 mg for 7 days; M, male; F, female; P25, 25th percentile; P75, 75th percentile.
Eradication rate by regimen group
In the H-VA group, 1 participant was lost to follow-up, and 1 participant was discharged due to a diagnosis of depression. In the L-VA group, 6 patients were lost to follow-up, 1 discontinued their participation, 1 was discharged due to cholecystectomy, and 2 participants were discharged due to adverse drug reactions. In the VAC group, 1 patient was lost to follow-up, 2 patients were discharged because they did not take the medicine as required, and 1 patient was discharged because of adverse drug reactions (Figure 1).
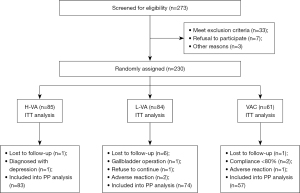
In ITT analysis, the efficacy of each regimen was 63.5% (54/85) in the H-VA group, 58.3% (49/84) in the L-VA group, and 60.7% (37/61) in the VAC group. In PP analysis, the eradication rate was 65.1% (54/83) in the H-VA group, 66.2% (49/74) in the L-VA group, and 64.9% (37/57) in the VAC group. According to the results of ITT analysis (χ2=0.032, P=0.786) and PP analysis (χ2=0.480, P=0.984), there was no significant difference in eradication rate among the 3 regimens (Table 2).
Table 2
| Regimen | H-VA | L-VA | VAC | P value |
|---|---|---|---|---|
| ITT | 63.5% (54/85) | 58.3% (49/84) | 60.7% (37/61) | 0.786 |
| PP | 65.1% (54/83) | 66.2% (49/74) | 64.9% (37/57) | 0.984 |
H-VA, vonoprazan 20 mg b.i.d with amoxicillin 750 mg q.i.d for 7 days; L-VA, vonoprazan 20 mg b.i.d plus amoxicillin 500 mg q.i.d for 7 days; VAC, vonoprazan 20 mg b.i.d plus amoxicillin 750 mg plus clarithromycin 500 mg for 7 days; ITT, intention to treat; PP, per protocol.
Security
All adverse effects were recorded after contacting patients by telephone. The adverse reaction rate was 16.90% in the H-VA group, 13.20% in the L-VA group, and 24.10% in the VAC group. Details of the adverse effects are shown in Table 3. According to χ2 test results, there was no significant difference in adverse reaction rate among the 3 groups (χ2=2.784, P=0.266). In the L-VA group, 1 case was discharged due to intolerable dizziness and nausea, and 1 case was discharged due to intolerable chest pain and sore throat. One case in the VAC group was discharged due to intolerable abdominal pain. All of the above symptoms disappeared after drug withdrawal.
Table 3
| Variables | Regimen | ||
|---|---|---|---|
| H-VA | L-VA | VAC | |
| Adverse effects (P=0.249) | 16.90% | 13.20% | 24.10% |
| Abdominal pain | 2 | 1 | 4 |
| Diarrhea | 1 | 0 | 1 |
| Abdominal distension | 5 | 1 | 6 |
| Nausea and vomiting | 4 | 5 | 2 |
| Dizziness | 2 | 1 | 0 |
| Generalized pain | 1 | 0 | 1 |
| Skin rash | 1 | 0 | 0 |
| Acid reflux | 3 | 1 | 3 |
| Feeling of hunger | 1 | 0 | 1 |
| Chest tightness | 0 | 2 | 0 |
| Bitterness in the mouth | 0 | 1 | 5 |
| Discontinuation of medication due to adverse effect | 0 | 2 | 1 |
| Compliance <80% | 0 | 0 | 2 |
H-VA, vonoprazan 20 mg b.i.d with amoxicillin 750 mg q.i.d for 7 days; L-VA, vonoprazan 20 mg b.i.d plus amoxicillin 500 mg q.i.d for 7 days; VAC, vonoprazan 20 mg b.i.d plus amoxicillin 750 mg plus clarithromycin 500 mg for 7 days.
Discussion
The increasing antibiotic resistance of H. pylori in China is the most important reason for the decline in the eradication rate. A cross-sectional observational study in China (15) revealed that the drug resistance rate of H. pylori was obviously higher in northwest China. This also poses a great challenge to the treatment of H. pylori in northwest China. However, amoxicillin has low drug resistance, and is the drug of choice for consideration in the eradication treatment of H. pylori in China. It is a time-dependent antibiotic, which can be quickly absorbed into plasma after administration and excreted in 6–8 hours. Compared with the frequency of administration twice a day in the guidelines, the efficacy may be more satisfactory after increasing the number of administrations (t.i.d or q.i.d).
During eradication therapy, maintaining the pH value in the stomach close to neutral is very important for the successful implementation of dual therapy, and the activity of H. pylori is closely related to the pH environment in the stomach. When the pH value in the stomach is 6–8, H. pylori will enter the reproductive state for the antimicrobial drugs to be effective. A study has shown that the survival and growth of H. pylori depend strictly on the pH in the stomach, so adequate acid inhibition is very important for eradicating H. pylori (16). Parallelly, the efficacy of antibiotics for H. pylori eradication is closely related to the pH value in the stomach. Amoxicillin, clarithromycin, and other antibiotics are pH-dependent, and the sensitivity of antibiotics increases when the pH value is >6. In 2015, Japan introduced a new gastric acid inhibitor, VPZ. VPZ is a kind of potassium-competitive acid blockers, which binds H+,K+-adenosine triphosphatase (ATPase) through reversible competition, thus inhibiting the secretion of gastric acid. Compared to PPIs, VPZ has a higher pKa value and is stable in acidic environment, and VPZ does not need acid activation. Therefore, compared with PPIs, VPZ can inhibit gastric acid secretion more strongly and persistently.
Therefore, the eradication regimen based on VPZ is expected to improve the eradication rate of H. pylori (6). The main enzyme involved in PPI metabolism is CYP2C19, and its gene polymorphism is an important factor affecting the pharmacokinetics of most PPIs. The efficacy of first-generation PPIs such as omeprazole in eradicating H. pylori is greatly affected by CYP2C19 genetic polymorphism. The genotypes of CYP2C19 were different among different races, with the Asian population being predominantly fast and moderate metabolizers. Some researchers have suggested that patients should be tested for CYP2C19 gene polymorphism during the first diagnosis and treatment to clarify whether they are fast metabolizers, so as to guide the selection of PPI or adjusted PPI agents such as rabeprazole that are less affected by CYP2C19 metabolism (17).
The dual therapy of PPI + amoxicillin was widely used in 1990s and was later replaced by triple therapy with more solid efficacy. In recent years, with the emergence of resistance problems to antibiotics such as clarithromycin, metronidazole, and levofloxacin, dual therapy has attracted attention again. After optimizing drug compatibility and adjusting the dose, high-dose dual therapy (HDDT) based on high-dose amoxicillin has highlighted its effectiveness in remedial therapy and first-line treatment of H. pylori. A HDDT regimen was co-administered by amoxicillin (1,000 mg 3 times/d or 750 mg 4 times/d) and PPI (standard dose 2–3 times/d or double standard dose 2 times/d) for 14 days (18). In China, due to the low rate of amoxicillin resistance, dual therapy may have more advantages for H. pylori eradication treatment.
At present, the fifth consensus on H. pylori infection in China (3) presents the eradication indications of H. pylori and suggests that all people diagnosed with H. pylori infection should receive treatment. Both VA dual therapy and VAC triple therapy have achieved a better eradication rate in Japan. Japanese guidelines have recommended VPZ as an alternative to PPI and candidate drugs to form a first-line H. pylori eradication regimen (19). The results of a meta-analysis which included 3 studies and 668 patients with H. pylori showed that the eradication rates of VA dual therapy in ITT and PP analysis were 87.5% and 89.6%, respectively. No significant differences were observed between VA dual therapy and VAC triple therapy. Researchers believe that VA dual therapy shows acceptable efficacy, good safety, and avoidance of unnecessary antibiotic use in the first-line treatment of H. pylori infection (20). However, the eradication regimens consisting of VPZ as an acid-suppressing drug have been less clinically tested in other countries and regions. Although VPZ has a stronger and longer-lasting acid acid-suppressing effect than PPI, it remains to be verified whether it can be used as the first choice of acid-suppressing drug for eradication of H. pylori in China, and there are still many issues to be explored in the regimens consisting of VPZ, such as drug dose, administration frequency, and duration. In a trial in the Jiangxi Province, the eradication rate was 89.2% for L-VA-10: amoxicillin (1,000 mg b.i.d.) and VPZ (20 mg b.i.d.) for 10 days; 81.1% for H-VA-10: amoxicillin (1,000 mg t.i.d.) and VPZ (20 mg b.i.d.) for 10 days; 66.7% for L-VA-7: amoxicillin (1,000 mg b.i.d.) and VPZ (20 mg b.i.d.) for 7 days; and 81.0% for H-VA-7: amoxicillin (1,000 mg t.i.d.) and VPZ (20 mg b.i.d.) for 7 days. The 4 therapies failed to achieve satisfactory eradication rates, and researchers believe that the duration of dosing should be extended to 14 days to improve the eradication rates (21). None of the 3 regimens investigated in this trial were shown to achieve a satisfactory eradication rate.
Previous studies have reported that the eradication regimens containing VPZ have a low adverse reaction rate and fewer severe adverse events, and the occurrence of adverse effects may be related to body mass index (BMI) and gender (22-24). The main adverse effects in this trial were abdominal distension (12 cases), nausea and vomiting (11 cases), abdominal pain (7 cases), and acid reflux (7 cases). The adverse reaction rate was not significantly related to height, weight, or gender. It was found that the symptoms of the adverse effect of acid reflux appeared 1 to 2 weeks after the patients stopped taking VPZ. Therefore, the use of VPZ in eradication therapy needs to be further explored to improve the symptoms of patients. At the same time, the adverse effect of symptoms of bitterness in the mouth mainly appeared in the VAC group, which may have been caused by the side effects of clarithromycin. A study has reported that patients with VPZ-containing eradication regimens will have side effects of influenza-like symptoms such as chest pain, generalized pain, and dizziness (25). In this trial, patients with the same symptoms appeared in all 3 groups of regimens, and 2 patients in the L-VA group were discharged because of intolerable adverse effects, and influenza-like symptoms resolved after discontinuation of the drug. The specific reasons need further study.
Since most of the participant in this trial were patients from the Gansu Province, China, the high drug resistance rate of patients in northwest China also makes eradication treatment very challenging. Even amoxicillin, which has a low drug resistance rate in other regions, still has 12.42% resistance in northwest China (13). After analyzing the clinical data of patients, the baseline data of patients (height, weight, smoking history, history of alcohol consumption, etc.) had no significant correlation with the eradication rate. Therefore, the high drug resistance rate is implicated as the main reason for the low eradication rate of the 3 therapies in this trial. In view of the current situation in northwest China, it is believed that neither VA nor VAC regimen can achieve satisfactory eradication rates. Although these 3 regimens have shorter dose types and dosing time, and better compliance and safety, they cannot be used as the first choice for eradication regimens. We believe that the following points can be improved: (I) extend the treatment time to 14 days; (II) if the treatment time is extended, the administration frequency q.i.d of amoxicillin may increase the difficulty of dosing. Under the premise of ensuring the dose (2 or 3 g/day), the administration frequency t.i.d may be more suitable; (III) add an antibiotic to form a triple therapy with VPZ and amoxicillin. Relevant studies suggest that rifabutin, sitafloxacin (26,27), and other antibiotics have low drug resistance rates, which may be a potential choice for patients with high drug resistance, but the administration method and safety still need to be further studied; and (IV) the use of bismuth and bismuth quadruple treatment for H. pylori infection is recommended in China’s guidelines, but relevant reports have not been seen on whether bismuth interacts with VPZ, and the eradication treatment of VPZ combined with bismuth. The eradication regimen of H. pylori needs to consider the different medical insurance coverage and drug supply between different regions and countries, as well as the drug resistance rate and cytochrome enzyme metabolism in different populations. Meanwhile, the eradication regimen suitable for patients in areas with a dual high prevalence of H. pylori infection and gastric cancer shall be explored. Large-sample and multicenter clinical trials are needed for in-depth exploration.
This study had some limitations. First, the sample size was limited to 214 patients with H. pylori infection from 3 centers in Gansu Province as a pilot study. Second, due to the objective conditions, the antibiotic resistance in patients who failed to eradicate was not analyzed, and a more reasonable regimen for remedial treatment could not be provided. Third, it was unclear if increasing the VPZ dosage (20 mg t.i.d.) would sustain higher pH levels and enhance the effectiveness of eradication regimens since the stomach pH was not evaluated during the course of treatment.
In conclusion, the 7-day eradication regimen consisting of VPZ as an acid-suppressing drug, whether it was a dual therapy combined with amoxicillin or a triple therapy combined with amoxicillin and clarithromycin, failed to achieve sufficient eradication rates, and the specific improvement methods require further exploration.
Acknowledgments
We thank No. 940 Hospital of Joint Service Support Force and Gansu Provincial People’s Hospital for contributing patients to the research cohort.
Funding: This study was supported by the Key R & D Program of Gansu Province (grant number 20YF8FA078); Key Talent Project of Gansu Province (Grant number 2022RCXM071).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4133/rc
Trial Protocol: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4133/tp
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4133/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4133/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology 2016;150:64-78. [Crossref] [PubMed]
- Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017;153:420-9. [Crossref] [PubMed]
- Liu WZ, Xie Y, Lu H, et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018;23:e12475. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020 Jul;70(4):313. [Crossref] [PubMed]
- Liou JM, Malfertheiner P, Lee YC, et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut 2020;69:2093-112. [Crossref] [PubMed]
- Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016;65:1439-46. [Crossref] [PubMed]
- Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of Vonoprazan for Helicobacter pylori Eradication. Intern Med 2020;59:153-61. [Crossref] [PubMed]
- Suzuki S, Gotoda T, Kusano C, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut 2020;69:1019-26. [Crossref] [PubMed]
- Lui RN, Chan FKL. Vonoprazan-containing therapy has an H pylori eradication rate >90% and increases eradication vs. standard triple therapy. Ann Intern Med 2021;174:JC117. [Crossref] [PubMed]
- Yang X, Li Y, Sun Y, et al. Vonoprazan: A Novel and Potent Alternative in the Treatment of Acid-Related Diseases. Dig Dis Sci 2018;63:302-11. [Crossref] [PubMed]
- Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol 2021;36:1159-63. [Crossref] [PubMed]
- Okubo H, Akiyama J, Kobayakawa M, et al. Vonoprazan-based triple therapy is effective for Helicobacter pylori eradication irrespective of clarithromycin susceptibility. J Gastroenterol 2020;55:1054-61. [Crossref] [PubMed]
- Miftahussurur M, Pratama Putra B, Yamaoka Y. The Potential Benefits of Vonoprazan as Helicobacter pylori Infection Therapy. Pharmaceuticals (Basel) 2020;13:276. [Crossref] [PubMed]
- Iannini PB. Cardiotoxicity of macrolides, ketolides and fluoroquinolones that prolong the QTc interval. Expert Opin Drug Saf 2002;1:121-8. [Crossref] [PubMed]
- Zhong Z, Zhang Z, Wang J, et al. A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter pylori strains in China. Am J Cancer Res 2021;11:5027-37. [PubMed]
- Auesomwang C, Maneerattanaporn M, Chey WD, et al. Ten-day high-dose proton pump inhibitor triple therapy versus sequential therapy for Helicobacter pylori eradication. J Gastroenterol Hepatol 2018;33:1822-8. [Crossref] [PubMed]
- Kagami T, Sahara S, Ichikawa H, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther 2016;43:1048-59. [Crossref] [PubMed]
- Hu Y, Zhu Y, Lu NH. Recent progress in Helicobacter pylori treatment. Chin Med J (Engl) 2020;133:335-43. [Crossref] [PubMed]
- Kato M, Ota H, Okuda M, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter 2019;24:e12597.
- Ouyang Y, Wang M, Xu YL, et al. Amoxicillin-vonoprazan dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. J Gastroenterol Hepatol 2022;37:1666-72. [Crossref] [PubMed]
- Hu Y, Xu X, Ouyang YB, et al. Optimization of vonoprazan-amoxicillin dual therapy for eradicating Helicobacter pyloriinfection in China: A prospective, randomized clinical pilot study. Helicobacter 2022;27:e12896. [Crossref] [PubMed]
- Furuta T, Yamade M, Kagami T, et al. Dual Therapy with Vonoprazan and Amoxicillin Is as Effective as Triple Therapy with Vonoprazan, Amoxicillin and Clarithromycin for Eradication of Helicobacter pylori. Digestion 2020;101:743-51. [Crossref] [PubMed]
- Bunchorntavakul C, Buranathawornsom A. Randomized clinical trial: 7-day vonoprazan-based versus 14-day omeprazole-based triple therapy for Helicobacter pylori. J Gastroenterol Hepatol 2021;36:3308-13. [Crossref] [PubMed]
- Eto H, Suzuki S, Kusano C, et al. Impact of body size on first-line Helicobacter pylori eradication success using vonoprazan and amoxicillin dual therapy. Helicobacter 2021;26:e12788. [Crossref] [PubMed]
- Gunaratne AW, Hamblin H, Clancy A, et al. Combinations of antibiotics and vonoprazan for the treatment of Helicobacter pylori infections-Exploratory study. Helicobacter 2021;26:e12830. [Crossref] [PubMed]
- Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol 2014;20:5283-93. [Crossref] [PubMed]
- Saito Y, Konno K, Sato M, et al. Vonoprazan-Based Third-Line Therapy Has a Higher Eradication Rate against Sitafloxacin-Resistant Helicobacter pylori. Cancers (Basel) 2019;11:116. [Crossref] [PubMed]
(English Language Editor: J. Jones)


