
Combination of Type I and II tyrosine kinase inhibitors—avapritinib and sunitinib—in refractory gastrointestinal stromal tumor after failure to multi-line therapy: a case report
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the digestive tract, and most commonly occur in the stomach (50–60%) or small intestine (30–40%). GISTs >2 cm in size are thought to have malignant potential (1,2). The liver and peritoneum are the most common sites of metastasis or recurrence after radical resection (3). Most GISTs develop due to KIT- or platelet-derived growth factor receptor alpha (PDGFRA)–activating mutations (70–80%). Surgery and tyrosine kinase inhibitors (TKIs) are the main treatment options (4).
Based on previous experience and evidence of therapy for advanced GIST patients, the sequential use of imatinib, sunitinib, and regorafenib is recommended as 1st-, 2nd-, and 3rd-line therapies, respectively (5). However, the long-term use of TKIs can lead to drug resistance, mainly due to outgrowth of clones with secondary resistant KIT mutations. These KIT secondary mutations are usually found in the adenosine triphosphate (ATP)-binding pocket encoded by exons 13 and 14, or the activation loop (A-loop) encoded by exons 17 and 18 (6,7). As a type II TKI, sunitinib has a good effect on the primary KIT exon 9 mutation and secondary KIT exon 13 and 14 mutations, and actively inhibits ATP-binding pocket mutations but not activation loop mutations (8,9). Additionally, sunitinib is also effective in anti-angiogenesis. Common adverse reactions of sunitinib include hematological toxicity, gastrointestinal reactions, diarrhea, proteinuria, hypertension and abnormal thyroid function, hand-foot syndrome, fatigue and so on. Conversely, avapritinib is a highly efficient and selective type I TKI that actively restrains KIT and PDGFRA activation loop mutants, especially KIT D816V and PDGFRA D842V (10). The most common adverse reactions (all grades) in avapritinib recipients were anemia, oedema, nausea, fatigue/asthenia, cognitive impairment and vomiting.
Lately before, a phase 1b/2a non-randomized clinical trial revealed that the combination of type I TKI (PLX9486) and type II TKI (sunitinib) had favourable efficacy and acceptable tolerability in the treatment of GIST. However, there is no effective case report of TKI combination therapy. In this article, we report a successful example of the combined application of a type I TKI and a type II TKI (i.e., avapritinib and sunitinib) in treating a patient who progressed from more than 4 standard-line treatments. This case provides a reference for the selective combination of TKIs in treating advanced GIST, and shows that precise combination therapy based on driving gene guidance should be considered in treating refractory imatinib-resistant advanced GISTs. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3746/rc).
Case presentation
In April 2018, a 55-year-old female attended a local hospital in Guangdong Province complaining of abdominal discomfort and distension for 2 months. The patient was diagnosed with an abdominal tumor and a computed tomography (CT) scan showed a mass of about 13.4 cm × 10.6 cm in the right abdominal cavity. In May 2018, the patient underwent surgery (R0 resection) at the local hospital. The postoperative pathology confirmed duodenal GIST, with a mitotic count <5/50 high power field (HPF). The immunohistochemistry showed CD117, CD34, DOG-1, and Ki-67 (5%) were all positive. Genetic testing revealed KIT_exon9_502_503dup.
The patient took 400 mg/d of imatinib after surgery and she attended routine examinations every 6 months. During regular follow up after 27 months after surgery (July 23, 2020), the CT scan suggested new metastases of the subphrenic peritoneum and abdominal seeding nodules, which indicated disease recurrence. A CT scan in September 2020 showed the enlargement of the former lesions with new abdominal and hepatic metastases that confirmed disease progression on imatinib, and the local hospital recommended the patient switch to sunitinib as 2nd-line therapy (37.5 mg/d continuously). After 5 months of sunitinib therapy, the liver (S7) metastases had become enlarged, but the other lesions remained stable (Figure 1). The patient complained of pain in the right upper quadrant, especially at night and when breathing deeply and was then referred to our center. Limited progressive disease of sunitinib from the liver (S7) lesion was considered.
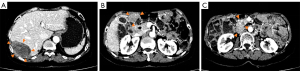
After a multi-disciplinary team discussion, the patient underwent a cytoreductive resection (R1) at our hospital on March 9, 2021. The postoperative pathology indicated the mitotic count of tissue from liver S7 was >50/5 mm2, and Ki-67 was 60% positive. The postoperative genetic testing of the resistant tumor tissue suggested that in addition to KIT exon 9 mutations, there were secondary genetic mutations, including KIT_exon16_p.L783V and TP53, POT1, SETD2 mutations. The preoperative circulating tumor DNA (ctDNA) was negative.
Given the failure of the sunitinib therapy, the patient switch to 3rd-line therapy with regorafenib from April 2021. Due to adverse reactions, mainly of significant HFS, the dose was gradually reduced from initial 160 to 80 mg/d. After 4 months of therapy, the CT scan suggested new lesions of the liver and peritoneum. The patient was confirmed disease progression of regorafenib. The ctDNA was still negative.
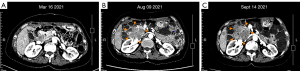
The patient then received standard 4th-line therapy of ripretinib (150 mg/d) on August 2021. After 1 month, the CT scan showed that the lesions were enlarged (Figure 2). At this time, all standard targeted therapies for GIST had failed, and no clinical trials were available. Given the poor inhibitory effect of ripretinib on the KIT exon 9 mutation, we recommended that the patient added extra sunitinib (25 mg/d) to the ripretinib (150 mg/d) as a combination therapy. After 18 days of taking this 5th-line therapy regimen, the CT scan on October 12, 2021 indicated that the main lesion beside the pancreatic head had slightly shrunk, and other lesions remained unchanged, which indicated a stable disease (SD) with tumor suppression effect. However, the adverse reactions, including fever (grade 1–2), HFS (grade 3), gum bleeding (grade 2), and hair loss (grade 1), were so significant that the patient refused to continue the dual-drug combination regimen.
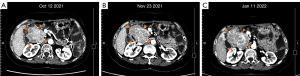
As the adverse reactions of the sunitinib and ripretinib combination therapy were mainly related to the sunitinib, we recommended that sunitinib be replaced with avapritinib. Thus, from October 12, 2021, the patient began taking avapritinib (300 mg/d) and ripretinib (150 mg/d) as 6th-line treatment. Due to the patient’s inability to tolerate adverse reactions and the appearance of capillary bleeding of fundus, the dosage was gradually reduced to 150 mg/d of avapritinib combined with 100 mg/d of ripretinib. After 3 months of therapy, the CT scan in January 2022 suggested that all the lesions were enlarged (Figure 3).
Taking the patient’s genotype with KIT exon 9 and 16 mutations and safety to various targeted drugs into consideration, we recommended that the patient received avapritinib (100 mg/d) plus sunitinib (37.5 mg/d) continuous daily dose (CDD) treatment as 7th-line regimen. Due to obvious adverse effects of taking avapritinib 150mg, she continued to apply 100mg of avapritinib in this new regimen and started on January 12, 2022. The patient could barely tolerate the side effects of this regimen, and experienced mild facial and left lower extremity edema (grade 1), moderate HFS (grade 2), and diarrhea (grade 2), but no myelosuppression. These adverse reactions caused the patient to discontinue the TKIs intermittently for 9 days and the patient resumed the drugs at the original dose after the symptoms had been relieved spontaneously.
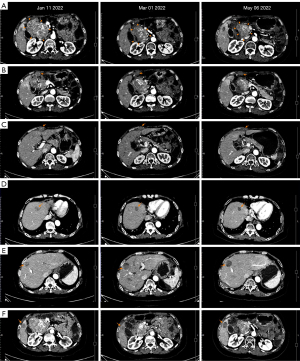
After about 40 days of administration, a CT scan on March 1, 2022, suggested that the diameter of the main lesion beside the head of the pancreas had reduced by 23% and the overall diameter of the target lesions had reduced by 15%. SD with tumor shrinking was determined. According to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria for 6 target lesions, including 3 peritoneal metastases and 3 liver metastases, and with the exception of the hepatic S8 metastasis that was slightly larger than before, the other metastatic lesions were all reduced compared to the previous measurement.
The patient maintained avapritinib and sunitinib dual-target regimen. In the next two months of treatment, the patient continued experiencing HFS (grade 2–3), double lower extremity edema (grade 2), diarrhea (grade 1) and developed leucocytopenia (grade 1), for which the patient adjusted the administered manner with short-term interruption according to intolerable side effects happened, usually in a way of 10 days on with 1–2 days off without altering dosage. In addition, the erythra could be relieved under the treatment of Ebastine. A CT scan in May 6, 2022 indicated that all the lesions were still responsive, and the main lesion was continuously shrinking by 30%. SD was determined based on the RECIST 1.1 criteria (Figure 4).
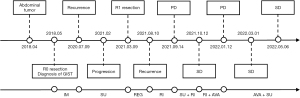
As a summary, we made a brief flowchart of the patient’s diagnosis and treatment process (Figure 5).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The advent of TKI has radically revolutionized the treatment of GIST (11). TKI is effective in patients with GIST. However, the development of drug resistance appears to be inevitable (12). The occurrence of resistance is mainly attributed to the acquisition of secondary gene mutations. These resistance mutations could be heterogeneous (e.g., may comprise multiple secondary mutations in different patients, or even different mutations in different parts from a single lesion) (13). An ideal multi-target inhibitor that covers all primary and secondary mutations has yet to be discovered.
Given its heterogeneity, single TKI targeted therapy can only inhibit some mutations, and the disease may continue to progress after multi-line sequential therapy, which has led to the exploration of TKI combination therapy to some extent (9). Before the appearance of ripretinib, a switch-control TKI active against a broad spectrum of KIT and PDGFRA mutations, there was a clinical study that tried to combine sunitinib and regorafenib with the purpose of achieving synergistic effect as the two TKIs could complimentarily inhibit different secondary mutations. Yet in consideration of safety, the study only used the form of taking the two TKIs for a short period of alternation, but not the form of taking the two TKIs at the same time. The end result showed an unsatisfactory efficacy and it was speculated the two TKIs both could not lead to the ability to achieve their respective effective tumor inhibition concentration in vivo when briefly alternating. This study revealed a brief sequential alternation of two TKIs may not be a reasonable combination therapy model (14). And there is a current study suggesting that the combination of type I and type II TKI may be a newly promising combination therapy idea for advanced GIST. To the best of our knowledge, there is no study or clinical report of combination therapies of TKI that have been approved for marketing. This article reported a successful case in which a type I TKI (avapritinib) and a type II TKI (sunitinib) were combined in clinical application.
Sunitinib is an oral multi-targeted TKI with anti-angiogenic and anti-tumor activities resulting from the blockade of several RTKs, including KIT, PDGFRs, and vascular endothelial growth factor receptors (VEGFRs) (15). Previous studies have shown that for imatinib-resistant or imatinib-intolerant GISTs of primary mutations, sunitinib has better efficacy in KIT/PDGFRA wild-type mutations and KIT exon 9 mutations than KIT exon 11 mutations, while for secondary mutations, sunitinib performs better for mutations of KIT exons 13 and 14 than that of KIT exons 17 and 18 (8,16).
Avapritinib is a potent and selective type I TKI of PDGFRA and KIT activation loop mutants and is approved for PDGFRA exon 18 (including D842V) mutant GIST (17,18). It also has the activity of anti-KIT exon 17 D816V mutations (19). Additionally, the anti-tumor activity of avapritinib on KIT exon 9 mutation (A502_Y503dup) is better than that of imatinib and comparable to that of sunitinib, which was revealed in the PDX model of a preclinical study (20). Thus, in addition to being effective against the GIST of the PDGFRA exon 18 mutations, avapritinib has a certain inhibitory effect on KIT exon 9 and 17 mutations.
In the genotypes of GIST, KIT exon 9 mutations with the majority of A502_Y503dup mutations account for about 10% of the incidence of GISTs, which mostly occur in the small intestine (21). Compared to the more common KIT exon11 mutation GISTs, both the postoperative relapse-free survival and subsequent tumor control time for each line of TKI therapy for KIT exon 9 mutation GISTs is short. Most GISTs with the KIT exon 9 mutation are sensitive to high-dose imatinib and sunitinib, but progression-free survival (PFS) is not necessarily satisfactory. In a subgroup analysis of a clinical trial comparing ripretinib and sunitinib as a 2nd-line treatment, it was shown that in the KIT exon 9 mutations, the PFS of ripretinib was inferior to that of sunitinib (22).
Previous studies have shown that TKI-resistant GISTs often have mutations in both the ATP-binding pocket and the A-loop, and the emergence of multiple drug-resistant sub-clonals, which limits the clinical activity of single TKI therapy (23-25). The absence of new, approved TKIs or available clinical trials makes the exploration of combination therapy of TKI possible. Drug combinations in advanced GISTs generally comprise a combination of two TKIs or a TKI and a downstream effect kinase inhibitor (26). A phase 1b/2a non-randomized clinical trial confirmed the efficacy of the combination of type I and type II TKIs in which PLX9486 and sunitinib inhibited the mutations and were safely co-administered at the recommended dose of both single agents in patients with refractory GISTs (27). For this patient, who had continuously progressed by multi-line targeted therapy with the primary KIT exon 9 mutation, we recommended the combination of a type I TKI, avapritinib, and a type II TKI, sunitinib, according to the driving gene. This regimen had the advantages of conformational and targeted complementarity and had a better tumor inhibitory effect than monotherapy. Judging from the current therapeutic effect, the combination effectively controlled multi-drug resistance. And this effect may lead to more rigorous therapeutic attempt, like another cytoreductive surgery. To better control the adverse reaction of combined targeted therapy, we recommend that personalized schedule should be considered. The patient could intermittently discontinue the treatment when side effect reached grade 3 or more, and resume the regimen after symptom relieved.
Finally, the combination of TKI therapies should focus on safety and seek to prevent more serious or intolerable adverse reactions. In this case, after the progression of sequentially targeted monotherapy, the combined treatment of ripretinib and sunitinib resulted in SD; however, the patient could not tolerate the obviously superimposed adverse reactions. The combination of TKIs has its unique advantages in inhibiting tumor progression, but it should also be noted that the combination may lead to a superposition of side effects. Thus, it is necessary to improve the survival outcomes of patients and maximize the efficacy of patients with GISTs under the premise of acceptable safety.
To the limitations, it should be pointed out that the single case report is not necessarily universally referential, and the follow-up duration of this case was short. The effective duration of the combination therapy of this case remained unclear.
Conclusions
In summary, the occurrence of drug resistance is common in the progression of GIST. When GIST is frequently advanced under single TKI targeted therapy, it can take into consideration the combination of different targeted drugs. However, attention should also be paid to assessing the safety of any combination therapy. Prospective clinical research is warranted to evaluate the safety and efficacy of different TKI combination therapies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3746/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3746/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol 2018;24:2806-17. [Crossref] [PubMed]
- Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol 2009;6:363-71. [Crossref] [PubMed]
- D'Ambrosio L, Palesandro E, Boccone P, et al. Impact of a risk-based follow-up in patients affected by gastrointestinal stromal tumour. Eur J Cancer 2017;78:122-32. [Crossref] [PubMed]
- Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): point mutations matter in management, a review. J Gastrointest Oncol 2017;8:466-73. [Crossref] [PubMed]
- Casali PG, Blay JY, Abecassis N, et al. Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2022;33:20-33. [Crossref] [PubMed]
- Nemunaitis J, Bauer S, Blay JY, et al. Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol 2020;16:4251-64. [Crossref] [PubMed]
- Yuzawa S, Opatowsky Y, Zhang Z, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 2007;130:323-34. [Crossref] [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. [Crossref] [PubMed]
- Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A 2009;106:1542-7. [Crossref] [PubMed]
- Dhillon S. Avapritinib: First Approval. Drugs 2020;80:433-9. [Crossref] [PubMed]
- Gross S, Rahal R, Stransky N, et al. Targeting cancer with kinase inhibitors. J Clin Invest 2015;125:1780-9. [Crossref] [PubMed]
- von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol 2018;36:136-43. [Crossref] [PubMed]
- Parab TM, DeRogatis MJ, Boaz AM, et al. Gastrointestinal stromal tumors: a comprehensive review. J Gastrointest Oncol 2019;10:144-54. [Crossref] [PubMed]
- Serrano C, Leal A, Phallen J, et al. Phase Ib study of rapid alternation of sunitinib (SU) and regorafenib (RE) in patients (pts) with advanced gastrointestinal stromal tumor (GIST). J Clin Oncol 2018;36:11510. [Crossref]
- Demetri GD, Garrett CR, Schöffski P, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res 2012;18:3170-9. [Crossref] [PubMed]
- Reichardt P, Demetri GD, Gelderblom H, et al. Correlation of KIT and PDGFRA mutational status with clinical benefit in patients with gastrointestinal stromal tumor treated with sunitinib in a worldwide treatment-use trial. BMC Cancer 2016;16:22. [Crossref] [PubMed]
- Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncol 2020;21:935-46. [Crossref] [PubMed]
- Jones RL, Serrano C, von Mehren M, et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: Long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur J Cancer 2021;145:132-42. [Crossref] [PubMed]
- Lübke J, Naumann N, Kluger S, et al. Inhibitory effects of midostaurin and avapritinib on myeloid progenitors derived from patients with KIT D816V positive advanced systemic mastocytosis. Leukemia 2019;33:1195-205. [Crossref] [PubMed]
- Gebreyohannes YK, Wozniak A, Zhai ME, et al. Robust Activity of Avapritinib, Potent and Highly Selective Inhibitor of Mutated KIT, in Patient-derived Xenograft Models of Gastrointestinal Stromal Tumors. Clin Cancer Res 2019;25:609-18. [Crossref] [PubMed]
- Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015;33:634-42. [Crossref] [PubMed]
- Heinrich MC, Jones RL, Gelderblom H, et al. INTRIGUE: A phase III, randomized, open-label study to evaluate the efficacy and safety of ripretinib versus sunitinib in patients with advanced gastrointestinal stromal tumor previously treated with imatinib. J Clin Oncol 2022;40:359881. [Crossref]
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [Crossref] [PubMed]
- Kim JJ, Ryu MH, Yoo C, et al. Phase II Trial of Continuous Regorafenib Dosing in Patients with Gastrointestinal Stromal Tumors After Failure of Imatinib and Sunitinib. Oncologist 2019;24:e1212-8. [Crossref] [PubMed]
- Serrano C, Mariño-Enríquez A, Tao DL, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer 2019;120:612-20. [Crossref] [PubMed]
- Klug LR, Khosroyani HM, Kent JD, et al. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol 2022;19:328-41. [Crossref] [PubMed]
- Wagner AJ, Severson PL, Shields AF, et al. Association of Combination of Conformation-Specific KIT Inhibitors With Clinical Benefit in Patients With Refractory Gastrointestinal Stromal Tumors: A Phase 1b/2a Nonrandomized Clinical Trial. JAMA Oncol 2021;7:1343-50. [Crossref] [PubMed]


