
Lung cancer with brain metastases remaining in continuous complete remission due to pembrolizumab and temozolomide: a case report
Introduction
Brain metastases (BM) are highly prevalent in non-small cell lung cancer (NSCLC), notably at diagnosis, and always indicate a fairly poor prognosis, with a median survival of less than 6 months among untreated patients (1-3). Although immunotherapy (in association or not to chemotherapy) has become an integral approach for advanced NSCLC treatment, the main therapeutic options for BM patients with NSCLC are surgical resection and radiation therapy (RT), which frequently include stereotactic radiosurgery and whole-brain radiation therapy (WBRT), accompanied by a risk of consequential neurotoxicity as high as 35% (4-8).
Anti-programmed cell death-1 (PD-1) therapy has been recommended as second-line therapy in NSCLC patients with progressive disease (PD) after a platinum-based regimen and a positive tumor proportional score (≥1%) (9). Although there have been many clinical trials of anti-PD-1 therapy for metastatic NSCLC patients, most have excluded such patients with untreated BM (10-14). To date, the only perspective phase II clinical trial of pembrolizumab for patients with untreated or progressive BM of melanoma and NSCLC had addressed the efficacy of pembrolizumab only for patients with BM, with a 33% intracranial objective response rate in 18 NSCLC patients. The enrolled patients with a BM size restriction between 5 mm and 20 mm were treated with pembrolizumab 10 mg/kg every 2 weeks until PD (15).
Chemotherapeutic drugs such as carboplatin combined with pemetrexed are beneficial for survival in BM patients with NSCLC (16), although their efficacy is greatly limited by the blood-brain barrier (BBB) and toxicity. Temozolomide (TMZ) is an oral alkylating agent with the characteristics of small molecular weight, wide antitumor spectrum, and lipophilicity (17). It has schedule-dependent activity in treating primary brain tumors and metastatic carcinoma and passes the BBB efficiently (18-20).
Immunotherapy, both as monotherapy and combination therapy with chemotherapy exert actions against BM in NSCLC patients (10,21-23). Pooled analysis of KEYNOTE-021, -189, and -407 retrospectively exploratory analyzed pembrolizumab plus platinum-based chemotherapy improved outcomes across all PD-L1 subgroups; even patients with BM achieved 18.8 months median overall survival (OS) yet only 7.6 months in chemotherapy alone (24). However, real world data in the ESCKEYP GFPC study revealed that there were no significant differences in response rates, progression-free survival, and OS in patients regardless of BM status with first-line immunotherapy (25). Varieties of retrospective clinical studies have revealed that immunotherapy combined with radiation therapy showed a survival benefit compared with exclusively systemic therapy in NSCLC BM patients (26,27). The best radiotherapy modality and chronological sequence to immunotherapy are still rarely reported. In addition, related studies on immunotherapy exacerbating radionecrosis were reported (28-30). Hence, optimal combination modalities with immunotherapy balancing on efficiency and safety in BM patients with NSCLC are urgently explored. Herein, we present a rare case of a 60-year-old male diagnosed with advanced NSCLC who achieved complete response (CR) for both primary lung lesions and multiple untreated BM after systematic combination therapy with pembrolizumab and oral temozolomide. Furthermore, with gradual and exploratory reduction of the pembrolizumab dose, the patient achieved continuous complete remission; the survival time has reached 59 months to date, with tolerable side effects. This RT sparing approach need to be confirmed in both preliminary studies and large randomized controlled trials. We present the following article in accordance with the CARE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4208/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standard of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s daughter. A copy of the written consent is available for review by the editorial office of this journal.
A 60-year-old Chinese man with a 40-pack-year smoking history presented to our hospital due to cough and expectoration in February 2017. No other symptoms were demonstrated. The patient did not have a family history of cancer or any other specific medical history, and his physical cardiopulmonary examination was normal. He had a body surface area of 1.89 m2 and an Eastern Cooperative Oncology Group (ECOG) score of 1. Chest computed tomography (CT) revealed a right middle lobe mass (2.7 cm × 2.1 cm) and metastasis in the right lower pulmonary hilum (5.0 cm × 6.2 cm), with mediastinal lymph node enlargement. Brain magnetic resonance imaging (MRI) showed multiple lacunar cerebral infractions in frontoparietal lobe. Immunohistochemical staining of a mediastinal lymph node biopsy confirmed lung adenocarcinoma, with CK7, TTF1, and Napsin A positivity, as examined by endobronchial ultrasonography. Immunohistochemistry indicated that the lung tumor cells was 95% positive for programmed death ligand-1 (PD-L1). Genetic analysis looked for the following gene status: EGFR, ROS1, ALK, BRAF, and C-MET. RET and NTRK were not examined. However, no driver mutations were detected. Based on the patient’s age, clinical manifestations, the results of imaging, histopathology, and immunohistochemistry of a mediastinal lymph node biopsy, the diagnosis of right NSCLC, stage IIIB (T4N2M0) was confirmed.
After carefully considering the risk and benefit of concurrent chemoradiation, the patient refused treatment with RT. Informed consent was provided by the patient in February 2017, and cisplatin combined with pemetrexed was administered as first-line chemotherapy. A partial response (PR) was achieved after 4 cycles of combination chemotherapy. Discussion with radiation oncologists was conducted to determine whether thoracic radiation therapy should be initiated immediately. Due to poor pulmonary function with bilateral pulmonary bullae and an increased tumor load, 2 more chemotherapeutic options were considered. Unfortunately, after the fifth cycle of chemotherapy, radiological evaluation showed PD. The regimen was changed to cisplatin plus gemcitabine as second-line chemotherapy starting in June 2017; however, after 1 cycle, the patient experienced hemoptysis and the radiological data indicated PD (Figure 1A).
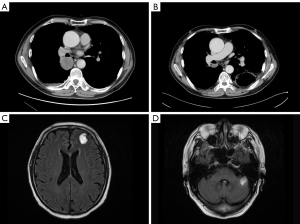
Thus, a concurrent regimen combining pembrolizumab and docetaxel was selected as third-line therapy from July 2017. After 2 cycles, a reduction in the size of the right lower pulmonary hilar mass was quickly confirmed by chest CT examination (Figure 1B). Brain MRI in September 2017 demonstrated multiple craniocerebral metastatic lesions in the left frontal lobe and cerebellum, with a maximum diameter of 2.1 cm × 1.5 cm (Figure 1C,1D). With dramatic hemoptysis relief and asymptomatic BM, pembrolizumab was continued; docetaxel was replaced with 250 mg of TMZ (132 mg/m2/d) per day for 5 days in October 2017. Although after 1 cycle treatment of TMZ combined with pembrolizumab, craniocerebral metastatic lesions in the left frontal lobe and cerebellum continued to shrink (Figure 2A,2B), a new metastasis was detected by MRI in the right occipital lobe, with a maximum diameter of 1.3 cm × 1.0 cm (Figure 2C). Without any discomfort, the patient remained on the combination therapy with TMZ plus pembrolizumab due to the continuous shrinking of both the lung lesions and BM (Figure 3A-3D). In January 2018, the patient achieved a CR for both the primary lung lesions and BM (Figure 3E-3H).
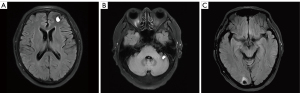
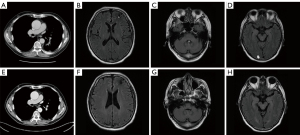
Pembrolizumab was then reduced to 100 mg per month with 250 mg of oral TMZ on days 1–5. The treatment was considered consolidation for half a year from February to August 2018, and monotherapy with pembrolizumab (100 mg per month) was maintained for another 24 months until July 2020.The patient currently remains alive with CR for both primary lesions and BM. The last follow-up time was in January 2022. Exudative inflammation in the left lower lobe was observed after the initial treatment of docetaxel with pembrolizumab, which was relieved with antibiotics instead of corticosteroids in September 2017. The patient experienced slight numbness on each side of his feet, which did not affect his daily life. We have summarized the clinical history and therapeutic process in Figure 4.

Discussion
We are reporting the first case of a patient with an advanced NSCLC with BM who achieved CR for both primary lung lesions and multiple untreated BM with pembrolizumab combined with systematic oral TMZ chemotherapy, with tolerable toxicity. Patient remains in remission with a normal quality of life as of January 2022, over 5 years from the original diagnosis of lung cancer and almost 5 years from the diagnosis of BM.
Overall, treatment for BM is challenging due to significant heterogeneity of the disease (31). Patients with a unique or a limited number of BM are considered eligible for treatment with complete surgical resection or stereotactic radiosurgery, and WBRT is preferably administered to patients with multiple BM (32). Complications following stereotactic radiosurgery such as radio necrosis and WBRT such as permanent neurocognitive impairment may outweigh the benefits (33,34). Therefore, RT sparing management has become increasingly attractive (35). Because of the limitation of the ability of platinum compounds to cross the BBB and target BM, immune checkpoint inhibitors (ICIs), which reactivate the T-cell immunity against tumor cells, have been used (36). In contrast to platinum-based chemotherapy, pembrolizumab, a highly selective humanized monoclonal IgG4-kappa isotope antibody against PD-1, has been shown to improve OS in patients with advanced or metastatic NSCLC without detectable driver gene mutations when administered as monotherapy or in combination with chemotherapy (11,12,23,37,38). In a study, an updated analysis of pembrolizumab for untreated BM patients or progressive BM patients with NSCLC after RT has revealed that pembrolizumab exerts activity in brain tumors at rates similar to those observed in extracranial tumors. The enrolled patients with BM between 5 and 20 mm in size were treated with pembrolizumab 10 mg/kg every 2 weeks until PD (13). In cohort 1, patients were tested for PD-L1 expression levels of at least 1% and achieved a 29.7% objective response rate (4 CR); in contrast, patients in cohort 2 showed PD-L1 expression less than 1% (or unevaluable) and had no BM response. The median time to BM responses was appropriately 1.8 months after the patients received a first or second assessment scan (13). In our case report, BM and lung lesion responses both occurred at the first disease assessment, which was approximately 2 months after the initiation of pembrolizumab, consistent with the above study. The first 5-year follow-up of a recent first-line immunotherapy trial for NSCLC demonstrated that 35 cycles of pembrolizumab at a dose of 200 mg once every 3 weeks (for nearly 2 years) were beneficial in 39 patients, with 82.1% of cases alive at the data cutoff (approximately 5 years) and without grade 5 treatment-related adverse effects; untreated BM patients were excluded (39). In our case, we used a dose reduced pembrolizumab (100 mg every 21 days) as maintenance therapy for over 2 years, indicating that lower doses might also be effective.
Due to its superior ability to cross the BBB, TMZ is approved for patients with glioblastoma (17). It has been reported that the concentration of TMZ in cerebrospinal fluid is only 20% of the serum concentration (40). Although a previous clinical trial showed that TMZ combined with WBRT led to a higher CR rate (38% vs. 33%; P=0.017) in patients with BM than WBRT alone, a higher occurrence of vomiting was observed in the combination group (41). The same results were observed in NSCLC patients with BM, with significant improvement in objective response rate and progression-free survival (6.0 vs. 3.5 months; P=0.038) with the TMZ+WBRT group, though the differences in OS were not significant (42). With regard to combination therapy with TMZ plus irinotecan or TMZ plus WBRT for BM originating from NSCLC, TMZ may control microscopic central nervous system disease (43,44). Nevertheless, TMZ combined with immunotherapy for BM patients is seldom used (45). As our patient had no central nervous system symptoms and the pulmonary hilar mass of the right lung was effectively controlled, addition of oral TMZ combined with pembrolizumab instead of WBRT and corticosteroids was recommended. Due to the lack of a safety profile regarding the dosage in NSCLC patients with BM, TMZ was administered under off-label use according to the oral dosing used in glioblastoma.
The efficacy of ICIs in NSCLC patients with BM was assessed in a prospective clinical trial by the Goldberg group, who found that ICIs have activity in BM after monotherapy with pembrolizumab (15). An updated analysis of 27 evaluable patients with PD-L1 expression >1% detected systemic responses for 3 cases of progressive BM disease (13). A similar manifestation was also observed in our patient, who showed progressive BM, even though the primary pulmonary lesions were reduced after treatment with pembrolizumab plus docetaxel. It should be noted that in that clinical trial, 2 of the 3 patients who had a PR in the body, but progressive BM, had a survival time of more than 2 years (13). Thus, it appears that a balance between immune surveillance and escape was achieved that prolonged the survivability of the tumor, rendering it difficult to eradicate completely or develop quickly. As our patient achieved continuous CR of both the primary tumors and BM after the combination therapy, we speculate that the effect of immunotherapy was probably synergistically augmented by changing the immune cells’ balance and increased immune surveillance induced by TMZ.
There is encouraging preliminary data to support the clinical trials in which immunotherapy alone or combined with RT deserve to be evaluated and patients with BM should not be excluded from active immune surveillance (35). However, preliminary evidence regarding the treatment of BM with TMZ in patients with NSCLC is rare (46). In one study, in a murine model of glioblastoma, dramatic chimeric antigen receptors (CARs) proliferation and enhanced persistence in the circulation were observed in the TMZDI pretreated group, as opposed to non-pretreated group where CARs showed poor expansion and survival in the circulation accompanied by PD in syngenetic and orthotopic gliomas (47). Moreover, CR was achieved for established brain tumor, as examined by bioluminescent imaging (47). In addition, TMZ exposure may affect DNA repair and result in selective cells with mismatch repair (MMR) inactivation which might increase the mutational load and promote continuous renewal of neoantigens in human colorectal cancers, as associated with response to ICIs (48-50). Moreover, TMZ may also reduce the number of circulating regulatory T cells rather than effector T cells in advanced melanoma patients, with a large number of activated effector T cells preserved in the tumor microenvironment (51,52).
In summary, monotherapy with immunotherapy alone or combined with RT in NSCLC patients with untreated BM is still under debate. As adverse events of RT may decrease quality of life in long-term survivors, ICIs combined with TMZ may be a well-tolerated, novel RT-sparing strategy for NSCLC patients with BM. The concrete mechanism of synergism and additivity between immunotherapy and TMZ in NSCLC BM is still uncertain. The limitation of these data is that it is an isolated case report, so preliminary evidence on the efficacy and safety of this strategy need to be confirmed to support development of more robust large randomized controlled trials.
Questions to be further discussed and considered
How to judge whether the new right occipital lobe metastases is a new locus or pseudoprogression after the first cycle of combination therapy of TMZ with pembrolizumab. If there is the very latest development in radiological examination or biomarkers to differentiate them besides pathological biopsy.
Paul Hofman: From my point of view it is more a new locus than a pseudoprogression development since a pseudoprogression usually developed from a preexisting already diagnosed lesion. Quite difficult to distinguish between pseudoprogression and tumor progression radiologically. To the best of my knowledge some artificial intelligence programs are under developed for trying to make this distinction? No biological biomarkers currently to the best of my knowledge.
Maciej M. Mrugala: It would not be a pseudoprogression if there was no lesion there previously. It is a very short period of time between the start of treatment and when the lesion was found. Advanced imaging such as PET scan or MRI perfusion could have been helpful. I understand none of the brain lesions were biopsied. I suggest we also review the initial scans where the strokes were found to make sure we are separating strokes from the presumed metastases.
In the clinical trial design part of KEYNOTE-021 and KEYNOTE-189, pembrolizumab plus chemotherapy were administered for up to 35 cycles. In our case, pembrolizumab was continuously maintained for nearly 5 years with good tolerance and free of recurrence. Whether such maintenance treatment with ICIs for BM make sense for a patient acquired CR.
Paul Hofman: There is no current international guidelines I think in this setting. So it can make sense if no associated toxicity is visible?
Maciej M. Mrugala: In the case report you indicate pembrolizumab was used for a little over 2 years, not 5 years: “The treatment was considered consolidation for half a year from February to August 2018, and monotherapy with pembrolizumab (100 mg per month) was maintained for another 2 years until July 2020. The patient currently remains alive with CR for both primary lesions and BM. The last follow-up time was in January 2022”. Please, clarify. Any conclusions based on case report have to be very cautious, so I would not elaborate any further here.
Various retrospective clinical studies have tried to provide evidence that ICIs may reverse the poor prognosis in NSCLC patients with BM. However, little has been done in those BM patients which were often excluded in clinical trials. How could we design a rather scientific and serious single-center small sample clinical trial of TMZ combined with ICIs for treatment of NSCLC with BM.
Paul Hofman: Definitively it is necessary to set up a prospective study with different arms including a control arm with the current gold standard treatment regimen.
Maciej M. Mrugala: Great idea to design a Phase II, single arm study for this indication. This is a discussion separate from this paper.
In this case, I think we should do even more, what other work should we perform to elaborate the concrete synergistical mechanisms of TMZ and ICIs, if we think so.
Paul Hofman: Any idea in the treatment of primary brain malignant tumors?
Maciej M. Mrugala: You could discuss more about the immune environment of the brain and specifically brain with metastases and provide some additional hypotheses why do you think there is synergy between pembrolizumab and TMZ.
Acknowledgments
We thank the patient’s daughter for purchasing pembrolizumab by flying to Macao every month in 2017 and thus permitting his inclusion in this study. The authors also appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was supported by the Natural Science Foundation of Hubei Province, China for 2021 (No. 2021CFB284).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-4208/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-4208/coif). PH received grant and contracts from Thermo Fisher Scientist (USA) and Biocartis (Belgium); consulting fees from Thermo Fisher Scientist, Biocartis, Amgen, Pfizer, AstraZeneca, Janssen, Roche, Eli Lilly and Abbvie; honoraria for speakers bureau from Thermo Fisher Scientist, Biocartis, Amgen, Pfizer, AstraZeneca, Janssen, Roche, Novartis, Bayer and BMS; support for travel from AstraZeneca, Janssen, Thermo Fisher Scientist and Biocartis. The other authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standard of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s daughter. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun YW, Xu J, Zhou J, et al. Targeted drugs for systemic therapy of lung cancer with brain metastases. Oncotarget 2018;9:5459-72. [Crossref] [PubMed]
- Raizer JJ, Hwu WJ, Panageas KS, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features. Neuro Oncol 2008;10:199-207. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Waqar SN, Morgensztern D, Govindan R. Systemic Treatment of Brain Metastases. Hematol Oncol Clin North Am 2017;31:157-76. [Crossref] [PubMed]
- Zakaria N, Satar NA, Abu Halim NH, et al. Targeting Lung Cancer Stem Cells: Research and Clinical Impacts. Front Oncol 2017;7:80. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Teixeira Loiola de Alencar V, Guedes Camandaroba MP, Pirolli R, et al. Immunotherapy as Single Treatment for Patients With NSCLC With Brain Metastases: A Systematic Review and Meta-Analysis-the META-L-BRAIN Study. J Thorac Oncol 2021;16:1379-91. [Crossref] [PubMed]
- Malhotra J, Jabbour SK, Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl Lung Cancer Res 2017;6:196-211. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 2020;21:655-63. [Crossref] [PubMed]
- Hendriks LEL, Henon C, Auclin E, et al. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J Thorac Oncol 2019;14:1244-54. [Crossref] [PubMed]
- Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016;17:976-83. [Crossref] [PubMed]
- Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol 2012;14:491-5. [Crossref] [PubMed]
- Schreck KC, Grossman SA. Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology (Williston Park) 2018;32:555-60, 569. [PubMed]
- Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 1987;47:5846-52. [PubMed]
- Danson SJ, Middleton MR. Temozolomide: a novel oral alkylating agent. Expert Rev Anticancer Ther 2001;1:13-9. [Crossref] [PubMed]
- Abrey LE, Christodoulou C. Temozolomide for treating brain metastases. Semin Oncol 2001;28:34-42. [Crossref] [PubMed]
- Gadgeel SM, Lukas RV, Goldschmidt J, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer 2019;128:105-12. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Powell SF, Rodríguez-Abreu D, Langer CJ, et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol 2021;16:1883-92. [Crossref] [PubMed]
- Descourt R, Greillier L, Perol M, et al. First-line single-agent pembrolizumab for PD-L1-positive (tumor proportion score ≥ 50%) advanced non-small cell lung cancer in the real world: impact in brain metastasis: a national French multicentric cohort (ESCKEYP GFPC study). Cancer Immunol Immunother 2022; Epub ahead of print. [Crossref] [PubMed]
- Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. [Crossref] [PubMed]
- Chen L, Douglass J, Kleinberg L, et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int J Radiat Oncol Biol Phys 2018;100:916-25. [Crossref] [PubMed]
- Kluger HM, Chiang V, Mahajan A, et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J Clin Oncol 2019;37:52-60. [Crossref] [PubMed]
- Alomari AK, Cohen J, Vortmeyer AO, et al. Possible Interaction of Anti-PD-1 Therapy with the Effects of Radiosurgery on Brain Metastases. Cancer Immunol Res 2016;4:481-7. [Crossref] [PubMed]
- Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016;125:17-23. [Crossref] [PubMed]
- Ernani V, Stinchcombe TE. Management of Brain Metastases in Non-Small-Cell Lung Cancer. J Oncol Pract 2019;15:563-70. [Crossref] [PubMed]
- Vilariño N, Bruna J, Bosch-Barrera J, et al. Immunotherapy in NSCLC patients with brain metastases. Understanding brain tumor microenvironment and dissecting outcomes from immune checkpoint blockade in the clinic. Cancer Treat Rev 2020;89:102067. [Crossref] [PubMed]
- Stockham AL, Ahluwalia M, Reddy CA, et al. Results of a questionnaire regarding practice patterns for the diagnosis and treatment of intracranial radiation necrosis after SRS. J Neurooncol 2013;115:469-75. [Crossref] [PubMed]
- Cheng H, Chen H, Lv Y, et al. Prospective memory impairment following whole brain radiotherapy in patients with metastatic brain cancer. Cancer Med 2018;7:5315-21. [Crossref] [PubMed]
- Eguren-Santamaria I, Sanmamed MF, Goldberg SB, et al. PD-1/PD-L1 Blockers in NSCLC Brain Metastases: Challenging Paradigms and Clinical Practice. Clin Cancer Res 2020;26:4186-97. [Crossref] [PubMed]
- El Rassy E, Botticella A, Kattan J, et al. Non-small cell lung cancer brain metastases and the immune system: From brain metastases development to treatment. Cancer Treat Rev 2018;68:69-79. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-Year Outcomes With Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50. J Clin Oncol 2021;39:2339-49. [Crossref] [PubMed]
- Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res 2004;10:3728-36. [Crossref] [PubMed]
- Antonadou D, Paraskevaidis M, Sarris G, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol 2002;20:3644-50. [Crossref] [PubMed]
- Zhu Y, Fu L, Jing W, et al. Effectiveness of temozolomide combined with whole brain radiotherapy for non-small cell lung cancer brain metastases. Thorac Cancer 2018;9:1121-8. [Crossref] [PubMed]
- Choong NW, Mauer AM, Hoffman PC, et al. Phase II trial of temozolomide and irinotecan as second-line treatment for advanced non-small cell lung cancer. J Thorac Oncol 2006;1:245-51. [Crossref] [PubMed]
- Han J, Qiu M, Su L, et al. Response and safety of whole-brain radiotherapy plus temozolomide for patients with brain metastases of non-small-cell lung cancer: A meta-analysis. Thorac Cancer 2021;12:3177-83. [Crossref] [PubMed]
- Khaddour K, Dowling J, Huang J, et al. Successful administration of sequential TVEC and pembrolizumab followed by Temozolomide in immunotherapy refractory intracranial metastatic melanoma with acquired B2M mutation. Oncotarget 2020;11:4836-44. [Crossref] [PubMed]
- Robins HI, Traynor AM, Mehta M. Temozolomide as prophylaxis for brain metastasis in non-small cell lung cancer. J Thorac Oncol 2006;1:732-3; author reply 733. [PubMed]
- Suryadevara CM, Desai R, Abel ML, et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 2018;7:e1434464. [Crossref] [PubMed]
- Cahill DP, Levine KK, Betensky RA, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res 2007;13:2038-45. [Crossref] [PubMed]
- Yip S, Miao J, Cahill DP, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res 2009;15:4622-9. [Crossref] [PubMed]
- Germano G, Lamba S, Rospo G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017;552:116-20. [Crossref] [PubMed]
- Ridolfi L, Petrini M, Granato AM, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med 2013;11:135. [Crossref] [PubMed]
- Banissi C, Ghiringhelli F, Chen L, et al. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother 2009;58:1627-34. [Crossref] [PubMed]
(English Language Editor: J. Jones)


