
Protocol for the development of a clinical practice guideline for discharge planning of stroke patients
Introduction
Data from the Global Burden of Disease (GBD) study show that in 2019, 12.2 million new cases of stroke occurred worldwide, with a total of 101 million people affected and 6.55 million deaths, accounting for 11.6% of all deaths and making stroke the second leading cause of death globally (1). From 1990 to 2019, the global disability-adjusted life expectancy due to stroke increased by 32% annually. In 2017, global direct and indirect costs due to stroke totaled US $891 billion, or 1.12% of the global GDP (2). On account of its “high incidence, high rate of disability, high mortality, high recurrence, and high economic burden,” stroke has become a worldwide problem that threatens human life and health (3).
Due to the disease characteristics of strokes, patients are often in the early stages of recovery at the time of discharge, with 75–86.5% of patients having varying degrees of physical dysfunction (4). This, together with the general lack of knowledge and skills in disease management, medication safety, and early rehabilitation among patients and caregivers, further affects the prognosis of patients via poor management after discharge, resulting in a high incidence of complications, increased risk of readmission, and even recurrent episodes, presenting a disease-model of stroke-re-stroke (disability)-re-stroke (death). A systematic evaluation (5) including 250,000 stroke patients showed that the 30-day readmission rate for stroke patients was 17.4%, and the one-year readmission rate was as high as 42.5%. The recurrence and readmission of stroke patients greatly affect their quality of survival and impose a heavy burden on them and their families. Therefore, meeting the needs of stroke patients in terms of medication guidance, rehabilitation guidance, and emotional and social support (6) ensures the continuity of medical care, and ensuring a smooth transition from the hospital to home has become an important issue that must be addressed.
Discharge planning refers to the process by which a hospital identifies the care needs of a patient for a smooth transfer from one health care facility to another setting and plans accordingly to help them complete appropriate treatment and care after discharge (7). A standardized discharge plan ensures that stroke patients receive continuous medical care when moving between settings and at home, thereby reducing complications, lowering readmission rates, and improving patient outcomes, which is important for reducing the burden of stroke (8). Despite a large and growing body of evidence demonstrating the significant role of discharge planning and discharge management in supporting stroke patients, and the growing number of primary researches and meta-analyses in this area, there is still a lack of standardized discharge plan guidelines for stroke patients. Currently, most existing international discharge planning guidelines have been developed for other diseases (9-11); they do not take into account the characteristics of stroke patients and the specific needs of their caregivers, and therefore provide only a limited reference for the implementation of discharge planning for stroke patients. Stroke-related guidelines focus more on prevention, treatment, and rehabilitation and less on discharge planning (12-14), thus they do not provide a systematic and comprehensive answer to the key issues of discharge planning and lack detailed recommendations for clinical application. Therefore, it is very necessary to develop a high-quality, evidence-based discharge planning guideline based on the growing and abundant evidence on the management of discharge planning in stroke patients, so as to further improve the quality of life of patients and their caregivers.
Therefore, our goal was to follow the methods and steps of the World Health Organization’s (WHO) guideline development manual to develop guidelines for stroke patient discharge planning by forming a multidisciplinary team to provide scientific, standardized, and detailed practical guidance to clinical health care providers, patients, and their caregivers, with the aim to promote the standardized management of stroke discharge planning and improve patients’ clinical outcomes.
Methods
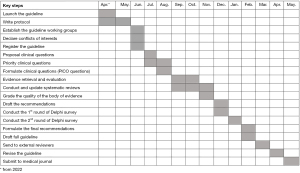
The guideline development process was designed to follow the WHO handbook for guideline development (15) and Guidelines 2.0: systematic development for a comprehensive checklist for a successful guideline enterprise (16). Evidence grading and guideline recommendations were based on the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (17). The guidelines were written with reference to the Reporting Items for Practice Guidelines in Healthcare (RIGHT) (18) and the Appraisal of Guidelines for Research and Evaluation (AGREE II) (19). This study of guideline development begins in April 2022 and ends in May 2023. The key steps and timeline of the guideline is shown in the Gantt chart (Figure 1).
Guidance sponsorship and support units
The guideline was initiated by the Evidence-based Nursing Center of West China Hospital, Sichuan University, with methodological support from the WHO Collaborating Centre for Guideline Implementation and Knowledge Translation.
Guideline registration and plan writing
The guideline was registered at the Practice guideline REgistration for transPAREncy (http://www.guidelines-registry.org/). The registration No. is IPGRP-2022CN331.
Guideline Project Group
The Guideline Project Group consists of the Guideline Steering Committee, the Guideline Consensus Expert Group, the Guideline Secretary Group, the Guideline Evidence Evaluation Group, the Guideline External Review Group, and the Guideline Conflict of Interest Management Committee, with the following specific memberships and responsibilities. Furthermore, the study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent will be obtained from all the participants.
Guideline Steering Committee
The Guideline Steering Committee is composed of five multidisciplinary experts with specialties ranging from clinical experts, nursing experts, health managers, and experts in evidence-based methodologies. The Guideline Steering Committee has a chairperson. The main responsibilities of the committee are (I) to define the topic and scope of each guideline; (II) to define the membership and work of other working groups; (III) to approve guideline protocol; (IV) to oversee the guideline development process; (V) to approve the recommendations and the full text of the guideline; and (VI) to provide advice and guidance for guideline development as necessary.
Guideline Consensus Expert Group
The Consensus Expert Group consists of 20–30 experts with the same multidisciplinary structure and increased participation of patient and caregiver representatives, with experts selected to be geographically, disciplinarily, and gender representative. The group’s main responsibilities are to (I) assess the importance of guideline questions and outcome indicators, (II) form recommendations on selected issues, (III) vote and consensus on recommendations, and (IV) finalize and promote the guideline.
Guideline Secretary Group
The Guideline Secretary Group includes two to five members who are staff from guideline initiating units with experience in guideline development. Its main responsibilities are (I) to coordinate the work of other working groups; (II) to draft the guideline protocol; (III) to conduct surveys and develop clinical key questions; (IV) to organize expert opinion consensus and discussion sessions; (V) to document the entire guideline development process; (VI) to write the first draft of the guideline; and (VII) to submit the guideline.
Guideline Evidence Evaluation Group
The Guideline Evidence Evaluation Group includes five to 10 members, consisting of experts in evidence-based methodology and personnel with experience in evidence-based practice, all with master’s or doctoral degrees and with systematic training in evidence-based medicine or evidence-based nursing practice. The key responsibilities of this group include (I) conducting literature and evidence searches, (II) completing evidence summaries and producing systematic evaluations, and (III) developing the GRADE evidence profiles and the summary of findings (SoF) tables (20,21).
Guideline External Review Group
The Guideline External Review Group includes five to 10 experts. After the full text of the guideline is completed, multidisciplinary experts who were not directly involved in the development of the guideline are invited to review the guideline with representatives of patients and their families. Their responsibilities include (I) reviewing the content and recommendations of the guideline and (II) ensuring the scientific accuracy, clarity, and impartiality of the guideline.
Conflict of Interest Management Committee
The Conflict of Interest Management Committee is composed of three members, including the Guideline Chair, the Methodologist, and a Managing Member. Its main responsibility is to collect, manage, update, and disclose the conflict of interest statements of all members of the guideline project team in accordance with the guideline’s conflict of interest management approach. The conflicts of interest of all members are disclosed as an annex to the full set of guidelines when it is published.
Scope of the guideline
The guideline focuses on stroke, including ischemic stroke and hemorrhagic stroke. The target audience includes neurological medical staff in hospitals at all levels. The target populations are stroke patients and their caregivers.
Collection of guideline questions
Relevant guidelines, systematic reviews, and original studies in the field of stroke discharge planning were systematically searched to refine and collect relevant clinical questions and outcome indicators. Questionnaires and interview outlines were also used to implement questionnaires and in-depth interviews with stakeholders, such as neurological medical staff and stroke patients and their families of the Guideline Project Group, to supplement the collection of relevant clinical questions and outcome indicators. Finally, based on the literature review and survey results, an alternative library of clinical key questions for the guideline was developed.
Identification of clinical problems and evaluation of their importance
Experts in the consensus group voted by consensus on the questions and corresponding outcome indicators that should be included in the guideline, and the importance of both clinical questions and outcome indicators were rated according to the GRADE system: a score of 7–9 indicated critical to decision making and recommendations; a score of 4–6 indicated important; and a score of 1–3 indicated less important. The guideline steering committee determined the final questions and outcome indicators to be included in the guideline based on the consensus results achieved through a consensus workshop.
Retrieval of evidence
The patient/population, intervention, comparison, and outcomes (PICO) framework was constructed for each guideline key question, the corresponding English and Chinese subject terms and free terms were identified separately, and search formulas were developed according to the search strategy of each data platform. The system searched the databases of PubMed, Embase, The Cochrane Library, CINAHL, ClinicalTrials.gov, the International Clinical Trial Registry Platform (ICTRP), the China Biology Medicine disc (CBM), Chinese National Knowledge Infrastructure (CNKI), and Wanfang Data. The search was limited to the period from construction of the library to August 2022, and no limitation was established on the language of publication to ensure that articles in any language were eligible for inclusion. The types of literature included systematic reviews, clinical controlled trials (randomized controlled trials, non-randomized controlled trials), and observational studies (cohort studies, case-control studies, and cross-sectional studies), and references were traced for the included literature to supplement the database search.
Evidence screening and data extraction
The literature inclusion and exclusion criteria were determined according to the PICO framework for each guideline question, de-weighting, reading the literature title, abstract, and full text order to screen the literature step-by-step, and collecting information for inclusion according to a pre-designed information extraction form. Each literature screening and information extraction step was performed independently by two investigators, and any disagreements were resolved by joint discussion or consultation with a third-party, evidence-based methodologist.
Evaluation of the quality of the literature
We used Assessing the Methodological Quality of Systematic Reviews 2 (AMSTAR 2) (22), the Cochrane Collaboration's tool for assessing risk of bias in randomized trials (23), and the Newcastle-Ottawa Scale (NOS) (24) to evaluate the methodological quality of the included literature. The evaluation process was completed independently by two researchers, and any disagreements were resolved by joint discussion or consultation with an evidence-based methodologist who consulted a third party.
Production and updating of systematic evaluations
For each guideline key question, according to the Cochrane Handbook for Systematic Reviews of Interventions (25), systematic reviews were developed by including original studies with a high-quality rating. If a high-quality systematic review published within three years existed for a key question, it was included directly; if the high-quality systematic review was published more than three years before, the systematic review was updated.
Grading the quality of evidence
Evidence quality grading was performed for the pooled evidence for each guideline question outcome indicator. Guideline questions that were included in the original research evidence support were graded according to the GRADE system, for five downgrading factors [limitations (risk of bias), imprecision, inconsistency, indirectness, and publication bias] and three escalating factors (large effect, dose response, and all plausible residual confounding). The final certainty of evidence will be graded as either high, moderate, low or very low (26). For guideline questions not supported by evidence, two rounds of expert correspondence were conducted by Delphi method to form expert consensus-based recommendations, namely Good Practice Statements (GPS) (27). The results of the evidence grading for each guideline key question were reported through the presentation of the GRADE evidence profiles (20) and the SoF tables (21).
Forming recommendations and reaching consensus
Based on the SoF tables, the values and preferences of the audience population and the cost of healthcare were combined to form recommendations according to the GRADE Evidence to Decision (EtD) frameworks (28,29). All the recommendations were integrated to form a recommendation letter questionnaire, which was distributed to the consensus group experts to evaluate and propose modifications, and four recommendation levels were given to the recommendations supported by evidence: strong, weak, strong against, and weak against (Table 1).
Table 1
| Strength level | Definition |
|---|---|
| Strong (I) | Support the use of an intervention where the benefits clearly outweigh the risks |
| Weak (II) | Support the use of an intervention where the benefits may outweigh the risks |
| Strong against (I) | Oppose the use of an intervention where the risks clearly outweigh the benefits |
| Weak against (II) | Oppose the use of an intervention where the risks may outweigh the benefits or the balance of benefits and risks is unclear |
GRADE, Grading of Recommendations Assessment.
External review of recommendations
After the recommendation consensus was adjusted, the external review group experts evaluated the clarity of the guideline statement and feasibility in clinical application, and the guideline steering committee adjusted the final recommendation of the guideline based on the feedback.
Writing, publishing, and updating the guideline
Based on RIGHT (18) and AGREE II (19), the guideline was written and published after approval and adoption by the Guideline Steering Committee. The guideline is updated every three to five years, while the guideline team closely monitors relevant newly published evidence and regularly adds, revises, and updates versions as needed.
Guideline dissemination, implementation, and evaluation
After publication, the guideline was introduced and disseminated through the self-media of the West China Hospital of Sichuan University and related societies, and relevant academic conferences. At the same time, a multicenter, large sample, randomized controlled trial was conducted to translate and implement the guideline to verify and evaluate their value for clinical guidance.
Discussion
Stroke is complicated by physiological, pharmacological, behavioral, and psychological interactions, even after treatment and discharge, with more than 70% of patients being left with varying degrees of functional impairment (30). This can take the form of aphasia, sensory impairment, swallowing dysfunction, cognitive impairment, and hemiparesis, and it is accompanied by poor daily life skills, poor quality of life, and an increased caregiver burden. Not only do stroke patients have a higher demand for knowledge, management, and social support, but caregivers also have a greater burden of care and stress due to the complexity of the disease, lack of knowledge, and inexperience (6). A study has shown that more than half of stroke patients’ family caregivers are likely to carry a caregiving burden, and caregivers of patients who have experienced a dysfunctional stroke bear 3.7 times the burden of care of caregivers of non-stroke patients (31). It is imperative to standardize the post-discharge management of stroke patients through guidelines to guide clinical practice in stroke patient discharge planning.
Currently, guidelines related to hospital discharge planning have been developed in the United States, the United Kingdom, and Canada, and the development of such guidelines is mostly organized by the government or institutions, such as associations with some degree of authority. The guidelines all highlight the need for multidisciplinary medical teams to work together to provide services, and some guidelines (9-11). Some clearly define the roles and responsibilities of the various professionals, while others provide specific processes and standardized tools for discharge planning, such as the guideline published by the National Health Service (NHS) in England (32), which sets out 10 steps to achieve timely discharge, and the guideline to transitional care issued by the Ontario Centre for Health Quality (11), which provides discharge screening, assessment tools, and a standardized discharge summary template. These guidelines can inform the implementation of discharge planning for stroke patients to some extent, but are less relevant because they do not consider the disease characteristics of stroke patients and the specific care needs and care demands of their caregivers. The clinical practice guidelines on stroke are more focused on prevention, treatment, and rehabilitation. Some of them are related to discharge planning; however, the number of pages is relatively small, and they lack process steps and implementation details (12-14). For example, the Canadian Best Practice Guidelines for Stroke Transition Management (12), which mentions assessing the needs of patients, families, and caregivers but does not recommend appropriate assessment tools; although it mentions training for caregivers, it does not elaborate on the timing, content, and precautions included in such training.
In fact, the timing of stroke discharge planning is unclear, the services are not systematic, and the evaluation tools are not uniform due to a lack of standardized stroke discharge planning guidelines. In terms of timing, the Italian Association of Hospital Cardiologists (ANMCO) recommends that all patients requiring a discharge plan have a discharge assessment and a discharge date within 24–48 hours of admission (33). In the UK, it is mandated that discharge risk screening be completed within 24 hours of patient admission and that the patient’s discharge be anticipated (32). However, many developing countries still do not specify when discharge planning should take place. In terms of service components, the Centers for Medicare and Medicaid Services (CMS) recommend referral services for discharged patients when implementing a discharge plan, as well as home visits and home environment modifications, depending on the patient’s circumstances (34). The CMS in the United States recommend that discharge programs be implemented with referral services and home visits and home environment modifications as appropriate. Due to the variability of medical settings and economic levels in developing countries, community development lags behind, making it difficult to ensure the continuity of stroke referral services. In terms of evaluation tools, a wide range of risk screening tools for stroke discharge are available, such as the Dutch Stroke Score (DSS), the Stroke Risk Assessment (SRA) (35), and the Readmission Stroke Screening Tool (RSST) (36). However, the evaluation criteria and scope of these tools are not uniform, and in addition, no assessment tools for stroke discharge needs are available. Therefore, there is an urgent need for high-quality stroke discharge planning guidelines to standardize the development, implementation, and evaluation of discharge plans.
This guideline will follow the clinical characteristics and management priorities of stroke and will be developed by a multidisciplinary guideline development team, in strict accordance with the core principles and methods of guideline development. They will address key issues in the implementation process of stroke discharge planning based on the best available clinical evidence, consider the values and wishes of patients and families, and combine the experience of clinical experts to provide answers, with a view to developing high-quality, practical clinical practice guidelines. It is believed that this guideline can provide an evidence-based reference for improving the quality of discharge services and standardizing the discharge management of stroke patients, ultimately improving their post-discharge rehabilitation and quality of life.
Acknowledgments
Funding: This work was supported by the Clinical and Translational Medicine Research Special Project of Chinese Academy of Medical Sciences (No. 2021-I2M-C&T-A-023), and the West China Nursing Discipline Development Special Fund Project, Sichuan University (No. HXHL19002).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3151/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study will be conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent will be obtained from all the participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol 2021;20:795-820. [Crossref] [PubMed]
- Owolabi MO, Thrift AG, Mahal A, et al. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health 2022;7:e74-85. [Crossref] [PubMed]
- Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res 2017;120:439-48. [Crossref] [PubMed]
- Chen WW, Gao RL, Liu LS, et al. China cardiovascular diseases report 2015: a summary. J Geriatr Cardiol 2017;14:1-10. [PubMed]
- Zhong W, Geng N, Wang P, et al. Prevalence, causes and risk factors of hospital readmissions after acute stroke and transient ischemic attack: a systematic review and meta-analysis. Neurol Sci 2016;37:1195-202. [Crossref] [PubMed]
- Moreland JD, Depaul VG, Dehueck AL, et al. Needs assessment of individuals with stroke after discharge from hospital stratified by acute Functional Independence Measure score. Disabil Rehabil 2009;31:2185-95. [Crossref] [PubMed]
- Bristow O, Stickney C, Thompson S. Discharge planning for continuity of care. League Exch 1976;i-viii, 1-144. [PubMed]
- Nunes HJ, Queirós PJ. Patient with stroke: hospital discharge planning, functionality and quality of life. Rev Bras Enferm 2017;70:415-23. [Crossref] [PubMed]
- Patient discharge [Internet]. UTMB Health; c2022 [cited 2022 Jun 14]. Available online: https://www.utmb.edu/policies_and_procedures/
- Discharge from hospital: pathway, process and practice [Internet]. Department of Health; c2022 [cited 2022 Jun 14]. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20130104194213/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4003252
- Adopting a common approach to transitional care planning: helping health links improve transitions and coordination of care [Internet].Health Quality Ontario; c2022 [cited 2022 Jun 14]. Available online: https://dokumen.tips/documents/adopting-a-common-approach-to-transitional-care-planning-adopting-a-common.html?page=1
- Cameron JI, O'Connell C, Foley N, et al. Canadian Stroke Best Practice Recommendations: Managing transitions of care following Stroke, Guidelines Update 2016. Int J Stroke 2016;11:807-22. [Crossref] [PubMed]
- Green TL, McNair ND, Hinkle JL, et al. Care of the Patient With Acute Ischemic Stroke (Posthyperacute and Prehospital Discharge): Update to 2009 Comprehensive Nursing Care Scientific Statement: A Scientific Statement From the American Heart Association. Stroke 2021;52:e179-97. [Crossref] [PubMed]
- Bryer A, Connor M, Haug P, et al. South African guideline for management of ischaemic stroke and transient ischaemic attack 2010: a guideline from the South African Stroke Society (SASS) and the SASS Writing Committee. S Afr Med J 2010;100:747-78. [Crossref] [PubMed]
- WHO handbook for guideline development, 2nd ed. World Health Organization; 2022 [cited 2022 Jun 14]. Available online: https://apps.who.int/iris/handle/10665/145714
- Schünemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ 2014;186:E123-42. [Crossref] [PubMed]
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94. [Crossref] [PubMed]
- Chen Y, Yang K, Marušic A, et al. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med 2017;166:128-32. [Crossref] [PubMed]
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42. [Crossref] [PubMed]
- Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol 2020;122:142-52. [Crossref] [PubMed]
- Carrasco-Labra A, Brignardello-Petersen R, Santesso N, et al. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. J Clin Epidemiol 2016;74:7-18. [Crossref] [PubMed]
- Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj 2017;358:j4008. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed ed. Chichester (UK): John Wiley & Sons; 2019.
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. [Crossref] [PubMed]
- Guyatt GH, Alonso-Coello P, Schünemann HJ, et al. Guideline panels should seldom make good practice statements: guidance from the GRADE Working Group. J Clin Epidemiol 2016;80:3-7. [Crossref] [PubMed]
- Alonso-Coello P, Schünemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [Crossref] [PubMed]
- Alonso-Coello P, Oxman AD, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016;353:i2089. [Crossref] [PubMed]
- Wang W, Jiang B, Sun H, et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017;135:759-71. [Crossref] [PubMed]
- Achilike S, Beauchamp JES, Cron SG, et al. Caregiver Burden and Associated Factors Among Informal Caregivers of Stroke Survivors. J Neurosci Nurs 2020;52:277-83. [Crossref] [PubMed]
- Achieving timely simple discharge from hospital [Internet]. National Health Service; c2022 [cited 2022 Jun 14]. Available online: https://www.bipsolutions.com/docstore/pdf/8092.pdf
- Mennuni M, Gulizia MM, Alunni G, et al. ANMCO Position Paper: hospital discharge planning: recommendations and standards. Eur Heart J Suppl 2017;19:D244-55. [Crossref] [PubMed]
- Cesta T. Centers for Medicare and Medicaid Services--new interpretative guidelines for the conditions of participation for discharge planning--part 2. Hosp Case Manag 2013;21:167-70. [PubMed]
- de Ridder IR, Dijkland SA, Scheele M, et al. Development and validation of the Dutch Stroke Score for predicting disability and functional outcome after ischemic stroke: A tool to support efficient discharge planning. Eur Stroke J 2018;3:165-73. [Crossref] [PubMed]
- Keawpugdee J, Silpasuwan P, Viwatwongkasem C, et al. Hospital Readmission Risks Screening for Older Adult with Stroke: Tools Development and Validation of a Prediction. Inquiry 2021;58:469580211018285. [PubMed]


