
Phospholipase A2 receptor is associated with hypercoagulable status in membranous nephropathy: a narrative review
Introduction
Membranous nephropathy (MN) is the most common cause of adult nephrotic syndrome (NS). The clinical manifestations of MN include massive proteinuria, hypoalbuminemia, prominent edema, and hyperlipidemia, and there are two manifestations: primary and secondary forms. In 25% of patients, the occurrence of MN is associated with underlying disease such as systemic lupus erythematosus and malignant tumor, exposure to certain drugs, or infection (viral, bacterial). Primary MN is diagnosed if no secondary cause is apparent (1). The main pathophysiology of MN consists of the deposition of immune complexes at the bottom of podocyte foot processes (2). Recent discovery of the phospholipase A2 receptor (PLA2R) represents a significant medical advance in our understanding of MN, as its presence serves as a basis for differentiating primary and secondary forms of the disease. However, coagulation cascade disturbances in patients with MN remain to be fully elucidated. Many studies (3-8) have shown that a high titer of PLA2R antibody aggravates proteinuria and hypoalbuminemia and predicts a lower likelihood of clinical remission in patients with PLA2R-associated MN. Factors such as proteinuria and hypoalbuminemia also increase the risk of thrombotic events. A study has shown that secretory phospholipase A2 (sPLA2) can promote the release of arachidonic acid (AA) from membrane phospholipids and serves as a high-affinity ligand for PLA2R (9). Furthermore, AA exhibits a close association with lipid metabolism and the coagulation process. Most of the previous studies focused on proteinuria and hypoalbuminemia, and there were few studies on the specific mechanism. In this review, we analyze the relationship between PLA2R and blood hypercoagulability in MN patients and discuss the potential role of sPLA2 in the disturbance of coagulation cascades in patients with PLA2R-related MN. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3572/rc).
Methods
China National Knowledge Infrastructure (CNKI) and PubMed searches were performed using the key words “arachidonic acid”, “coagulation disorder”, “membranous nephropathy”, “PLA2R”, and “sPLA2” to identify relevant articles published in multiple languages and fields. We searched all types of relevant literature from 1991 to 2021, many related literatures were retrieved and abstract and introduction information was retrieved. After familiarizing ourselves with the research status at home and abroad, we formulated preliminary research ideas and further screened and consulted literatures. Ultimately, 47 relevant articles were selected. We classified and summarized important information before drawing our own conclusions and offering a new standpoint (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | 2021.05.01–2021.07.01 |
| Databases and other sources searched | CNKI, PubMed |
| Search terms used (including MeSH and free text search terms and filters) | Arachidonic acid, coagulation disorder, membranous nephropathy, phospholipase A2 receptor, secretory phospholipase A2 |
| Timeframe | 1991–2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | All types of articles were included in the analysis |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | All authors refer to relevant articles, and those with different opinions focus on discussion |
Discussion
The main complications of NS include infection, thromboembolic disease, acute kidney injury and protein metabolism disorder. Venous thromboembolism (VTE) is a common and serious complication of NS and includes deep vein thrombosis (DVT), renal vein thrombosis (RVT), and pulmonary embolism (PE). MN is the most common type of NS with thromboembolism (10), in which the incidence of RVT can be as high as 40% to 50% (10). While the process responsible for the hypercoagulable state in patients with MN remains to be elucidated, recent study has shown that syndecan-1 (SDC-1) may be associated with hypercoagulability and thromboembolism in patients with the disease (11).
PLA2R antibody has high specificity and sensitivity. In patients with PLA2R antibody associated IMN, serum PLA2R antibody level and PLA2R expression in kidney tissue were significantly increased (12). A study (13) has shown that the serum positive rate of PLA2R antibody in IMN patients is 70–80%. Other study (14) has shown that PLA2R can be continuously expressed in protocellular cells, and the expression of PLA2R can be detected in renal tissues of about 75% IMN patients. Multiple studies (15,16) have confirmed that the expression of PLA2R in renal tissue is expressed on protocellular surface through intracellular recycling, and is not affected by changes in patients’ conditions, so its diagnosis of IMN has a high stability. Studies (17,18) have shown that the risk of renal function progression increases with the increase of PLA2R antibody levels. Therefore, PLA2R is closely related to the diagnosis, treatment, occurrence and development of IMN.
However, the relationship between PLA2R and hypercoagulability in MN remains unclear. Current research suggests that with the loss and compensatory increase in the synthesis of urinary protein, changes in a variety of coagulation factors and associated regulators involved in coagulation and fibrinolysis occur, and in combination, these changes contribute to a prothrombotic environment (10). Other pathological characteristics of MN, in combination with the concomitant use of diuretics and immunosuppressive agents, may further increase the risk of thrombosis in these patients.
PLA2R antibody binds to podocyte PLA2R antigens, leading to the formation of membrane attack complexes that include podocytes, resulting in cytotoxicity with filtration barrier disruption and the development of proteinuria. Many studies (3-8) indicated PLA2R antibody titers were positively correlated with proteinuric levels and negatively correlated with serum albumin (Table 2). Serum albumin is an important determinant that assists in evaluating whether patients with MN need anticoagulation. Those with a high PLA2R antibody titer frequently have lower serum albumin, placing them at a greater risk of thrombotic events than those with negative PLA2R antibody or those with a low antibody titer. Other studies (4,19-23) showed spontaneous remission rates were lower among PLA2R antibody positive patients than those with a negative PLA2R (Table 3). A meta-analysis (24) of 2,345 patients from 29 cohorts showed difficulty in achieving remission is synonymous with a more severe and protracted course of proteinuria and hypoalbuminemia, placing patients at an increased risk of thrombosis. In addition, we found in a literature review that PLA2R may further aggravate blood hypercoagulation in MN patients through sPLA2 and AA pathways. Therefore, it is important to elucidate the risk and associated mechanism responsible for thrombotic events in patients with PLA2R-associated MN.
Table 2
| Author | Study design | Biochemical indicators | N: outcome |
|---|---|---|---|
| Antonella Radice et al. (3) | Prospective study in patients with PLA2R-related MN | Proteinuria; Alb; PLA2R-ab titer | 42: the positive PLA2R serum antibody status was linearly associated with increasing proteinuria and decreasing serum albumin over time, compared with negative antibody status |
| Zhang Qiuhua et al. (4) | Prospective study in patients with PLA2R-related MN | Proteinuria; Alb; PLA2R-ab titer | 552: patients with high serum PLA2R-ab drops had significantly lower Alb (30.93 vs. 35.80 g/L, P=0.048) and higher 24-hour proteinuria (2.34 vs. 1.22 g/day, P=0.031) than those with low titers |
| Qu Zhen et al. (5) | Prospective study in patients with PLA2R-related MN | Proteinuria; Alb; PLA2R-ab titer | 359: the level of PLA2R-ab was positively correlated with urine protein (r=0.164, P=0.002) and negatively correlated with Alb (r=−0.229, P<0.001) |
| Elion Hoxha et al. (6) | Prospective study in patients with PLA2R-related MN | Proteinuria; Alb; PLA2R-ab titer | 133: the decrease of serum PLA2R-ab level is associated with the decrease of proteinuria and the increase of Alb |
| Han Wenwen et al. (7) | Prospective study in patients with PLA2R-related MN | Proteinuria; PLA2R-ab titer | 56: there was a significant positive correlation between PLA2R-ab titer and proteinuria during treatment |
| Jyun Ni Chi et al. (8) | Prospective study in patients with PLA2R-related MN | Proteinuria; PLA2R-ab titer | 72: the titer of PLA2R-ab antibody was positively correlated with the severity of proteinuria (r=0.3227, P=0.03) |
PLA2R, phospholipase A2 receptor; ab, antibody; Alb, serum albumin; MN, membranous nephropathy.
Table 3
| Author | Study design | Cutoff value, N | Outcome |
|---|---|---|---|
| Zhang Qiuhua et al. (4) | Prospective study in patients with PLA2R-related MN | 0.91 mg/L, 552 | The clinical remission rate (51.22 vs. 82.35%, P=0.027) and complete remission rate (47.62% vs. 64.29%) in patients with high titer were also significantly lower than those with low titer |
| Sjoerd A. M. E. G. Timmermans et al. (19) | Prospective study in patients with PLA2R-related MN | 2 RU/mL, 73 | Patients with low PLA2R-ab had the highest rate of spontaneous remission (79%) |
| Piero Ruggenenti et al. (20) | Prospective study in patients with PLA2R-related MN | 14 RU/mL, 154 | Lower PLA2R-ab titers strongly predicted higher remission rates and shorter remission times. After initial reduction or complete depletion, PLA2R-ab titers increase after initial reduction or complete depletion, strongly indicating recurrence of the disease |
| Franck Pourcine et al. (21) | Retrospective study in patients with PLA2R-related MN | 14 RU/mL, 108 | Patients who had cleared PLA2R-ab from serum within 6 months had a greater chance of clinical remission than those who remained serologically positive |
| Elion Hoxha et al. (22) | Prospective study in patients with PLA2R-related MN | 14 RU/mL, 37 | Patients with PLA2R-ab negative MN had high rates of spontaneous proteinuria remission |
| Dan Dong et al. (23) | A meta-analysis about PLA2R-related MN | N=1,642 | Remission rate in the serum anti-PLA2R antibody-positive group was lower than that in the -negative group (OR =0.41, 95% CI: 0.28, 0.61, P<0.00001) |
PLA2R, phospholipase A2 receptor; ab, antibody; MN, membranous nephropathy.
sPLA2 has been shown to serve as a high-affinity ligand for PLA2R, promoting the release of AA from membrane phospholipids in mice (25). AA has been linked to lipid metabolism and blood clotting, and below, we propose potential roles for sPLA2 and AA in coagulation disorders among patients with PLA2R-related MN.
sPLA2, in combination with PLA2R, induces podocyte apoptosis
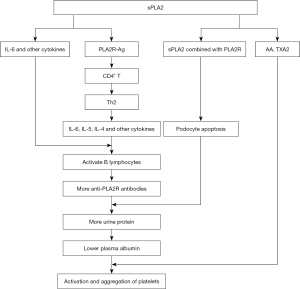
sPLA2 is a ligand with high affinity for PLA2R (25), which regulates multiple biological effects of the former. sPLA2-IB, an endogenous PLA2R ligand, induces cell proliferation, as well as the migration and production of lipid mediators (26). Using a mouse model, Beck et al. (27) found the expression of sPLA2-IB and its receptor in the kidney increased significantly. Another study (21) of 245 patients showed the level of circulating sPLA2-IB in idiopathic and secondary MN was significantly higher than in the control group, and sPLA2-IB levels were negatively correlated with serum albumin while positively correlated with 24-hour urinary protein amount. The role of sPLA2-IB in the diagnosis of both idiopathic and secondary MN remained undetermined in their study, but others (28) showed podocytes transfected with a PLA2R plasmid exhibited higher levels of apoptosis in response to sPLA2-IB treatment. These findings suggest sPLA2-IB may induce human podocyte apoptosis by binding with PLA2R. The sPLA2-IB-PLA2R interaction stimulates podocyte apoptosis by activating ERK1/2 and cPLA2 and increasing podocyte AA content. In patients with MN, the apoptosis rate of podocytes increased in groups with higher PLA2R expression and increased serum sPLA2-IB levels, while groups with higher PLA2R expression had lower serum albumin levels and higher urine protein levels (28). The pathogenesis of PLA2R-related MN mainly consists of three steps: the formation of PLA2R antigen-antibody immune complexes activate the complement system, allowing membrane attack complexes to enter cells, resulting in cellular toxicity and destroying the filtration barrier. Finally, patients develop proteinuria. sPLA2-IB may induce podocyte apoptosis by binding with PLA2R, further increasing proteinuria and exacerbating hypoalbuminemia in patients with MN. Abnormal platelet activation and aggregation in patients with NS may also increase platelet counts and promote the release of active substances. As albumin inhibits the production and release of AA and thromboxane A2 (TXA2), hypoalbuminemia in patients with NS increases their platelet count, while the activation and aggregation of platelets (29) exacerbates coagulation disorders and hypercoagulability in patients with PLA2R-related MN.
sPLA2 may promote the production of anti-PLA2R antibodies
A new study indicates the percentage of circulating plasma cells and regulatory B cells (Breg) in patients with MN is significantly higher than patients with non-immune-mediated chronic kidney disease and healthy controls (30). Anti-PLA2R antibodies are typically of the IgG4 subtype (1), and the production of IgG4 in patients with MN requires prior antigen presentation to CD4+ T-cells, which transform into corresponding T-helper cells 2 (Th2). Th2 cells and their secreted cytokines, including interleukin-4 (IL-4), IL-5, IL-6, and IL-10, activate B lymphocytes and generate specific IgG4 (31-33). In addition to enzymatic hydrolysis, sPLA2 has many effects, including the induction of cell proliferation, migration, hormone release, the production of lipid mediators, and cytokines, and relevant signal transduction. These effects are generated after sPLA2 binds to PLA2R. In the kidney, sPLA2 increases the expressions of PLA2R antigens by upregulating PLA2R expression (28), and this facilitates antigen presentation and increases the production of anti-PLA2R antibody. In addition, AA metabolism can produce many factors that regulate lipids, such as prostaglandin, thromboxane, interleukin, and lipoxygenin (34,35). Previous study has shown sPLA2 can activate cytokines such as tumor necrosis factor (TNF), IL-1, and IL-6 (36), which promote B-cell activation and increase antibody production, while other studies (7,12,37) also showed levels of serum PLA2R were proportional to those of urine protein antibody. Thus, by increasing the production of anti-PLA2R antibodies, sPLA2 exacerbates hypoproteinemia, further stimulating the activation and aggregation of platelets, leading to coagulation disturbance and, ultimately, to blood hypercoagulability in patients with PLA2R-related MN.
sPLA2 may promote the release of AA from membrane phospholipids
sPLA2 is a phospholipid polymerase and a ligand that can affect cellular survival. sPLA2 belongs to the PLA2 superfamily of enzymes, which catalyze the hydrolysis of sn2 ester bonds in glycerolipid, releasing free fatty acids and hemolytic lecithin (38). Therefore, AA-containing phospholipids can be hydrolyzed by PLA2, producing free AA and hemolytic lecithin (9). In the kidney, sPLA2-IB stimulates cPLA2a phosphorylation in podocytes through activation of the ERK1/2 pathway and increases AA production by upregulating PLA2R (28).
AA induces platelet aggregation
Vascular obstruction related to thrombosis is often due to uncontrolled platelet over-activation, leading to platelet dysfunction (39,40). Platelets are the smallest type of blood cells and play an important role in blood circulation, vascular integrity, and hemostasis (41). Excessive systemic platelet aggregation leads to thrombus formation and even acute coronary syndrome (ACS) (42). AA is a precursor of thrombin and prostacyclin compounds that is capable of regulating platelet function (43). Platelet aggregation is mediated by the synthesis of prostaglandin G2, prostaglandin H2, and TXA2, which are derived from the catalytic effects of cyclooxygenase (COX) on AA released from platelet membranes. These aggregates lead to increased intracellular Ca2+ mobilization through the inhibition of nitric oxide (NO) synthesis. Ca2+ released from dense granules in platelet cytoplasm can activate sPLA2, releasing AA from platelet membrane phospholipids (44). AA produces TXA2 (45) under the action of COX-1 and TXA synthase, leading to greater platelet aggregation. A study has shown complement C5B-9 damages glomerular epithelial cells by releasing AA,further aggravating hypoalbuminemia and hypercoagulability (46).
Conclusions
In summary, the discovery of PLA2R and its pathogenic role is an important milestone in the study of MN. Previous studies have clarified the pathogenesis, diagnosis, treatment, and prognosis of patients with PLA2R-related MN, but few have addressed the origin of the hypercoagulable state and the disturbed coagulation cascade. As a high-affinity ligand for PLA2R, sPLA2 plays an important role in complications associated with MN, and the serum titer of anti-PLA2R increases in patients with PLA2R-related MN. Based on the three lines of evidence outlined above, we infer sPLA2 may promote hypercoagulability in patients with PLA2R-related MN (Figure 1). The limitation of this finding is the lack of data support, and we are conducting a prospective study to explore this mechanism. At the same time, additional studies are required to elucidate the mechanisms responsible for the disturbed coagulation cascades in patients with PLA2R-related MN.
Acknowledgments
We greatly appreciate the Health Commission of Jilin Province for the grant.
Funding: This work was supported by the Health Commission of Jilin Province (Project No. 2020J112).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3572/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3572/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoxha E, Kneißler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 2012;82:797-804. [Crossref] [PubMed]
- Beck LH Jr, Salant DJ. Membranous nephropathy: recent travels and new roads ahead. Kidney Int 2010;77:765-70. [Crossref] [PubMed]
- Radice A, Trezzi B, Maggiore U, et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun Rev 2016;15:146-54. [Crossref] [PubMed]
- Zhang Q, Huang B, Liu X, et al. Ultrasensitive Quantitation of Anti-Phospholipase A2 Receptor Antibody as A Diagnostic and Prognostic Indicator of Idiopathic Membranous Nephropathy. Sci Rep 2017;7:12049. [Crossref] [PubMed]
- Qu Z, Zhang MF, Cui Z, et al. Antibodies against M-Type Phospholipase A2 Receptor May Predict Treatment Response and Outcome in Membranous Nephropathy. Am J Nephrol 2018;48:438-46. [Crossref] [PubMed]
- Hoxha E, Thiele I, Zahner G, et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 2014;25:1357-66. [Crossref] [PubMed]
- Han WW, Tang LJ, Kong XL, et al. Clinical significance of autoantibodies in the assessment and treatment of idiopathic membranous nephropathy. Exp Ther Med 2019;17:1825-30. [PubMed]
- Chi JN, Lai TS, Wu CF, et al. The relationship of anti-phospholipase A2 receptor antibody and C5a complement with disease activity and short-term outcome in idiopathic membranous nephropathy. J Formos Med Assoc 2019;118:898-906. [Crossref] [PubMed]
- Triggiani M, Granata F, Giannattasio G, et al. Secretory phospholipases A2 in inflammatory and allergic diseases: not just enzymes. J Allergy Clin Immunol 2005;116:1000-6. [Crossref] [PubMed]
- Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol 2012;7:513-20. [Crossref] [PubMed]
- Chen X, Geng X, Jin S, et al. The Association of Syndecan-1, Hypercoagulable State and Thrombosis and in Patients With Nephrotic Syndrome. Clin Appl Thromb Hemost 2021;27:10760296211010256. [Crossref] [PubMed]
- Yuan WM, Cheng GY, Li YS, et al. The value of PLA2R and IgG4 in the diagnosis of primary membranous nephropathy in the elderly. Chinese People's Liberation Army Medical Journal 2017;42:810-4.
- Lv J, Hou W, Zhou X, et al. Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J Am Soc Nephrol 2013;24:1323-9. [Crossref] [PubMed]
- Wang LJ, Shang MH, Zhuge YF, et al. Expression of PLA2R in renal tissue of patients with idiopathic membranous nephropathy and its relationship with therapeutic effect of immunotherapy. Chinese Medical Journal 2016;96:4-8. [PubMed]
- Wen J, Xie K, Zhang M, et al. HLA-DR, and not PLA2R, is expressed on the podocytes in kidney allografts in de novo membranous nephropathy. Medicine (Baltimore) 2016;95:e4809. [Crossref] [PubMed]
- Ge YC, Jin B, Zeng CH, et al. PLA2R antibodies and PLA2R glomerular deposits in psoriasis patients with membranous nephropathy. BMC Nephrol 2016;17:185. [Crossref] [PubMed]
- Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 2012;23:1735-43. [Crossref] [PubMed]
- Kanigicherla D, Gummadova J, McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 2013;83:940-8. [Crossref] [PubMed]
- Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW, et al. Anti-PLA2R Antibodies as a Prognostic Factor in PLA2R-Related Membranous Nephropathy. Am J Nephrol 2015;42:70-7. [Crossref] [PubMed]
- Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-Phospholipase A2 Receptor Antibody Titer Predicts Post-Rituximab Outcome of Membranous Nephropathy. J Am Soc Nephrol 2015;26:2545-58. [Crossref] [PubMed]
- Pourcine F, Dahan K, Mihout F, et al. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: A single-centre study over 14 years. PLoS One 2017;12:e0173201. [Crossref] [PubMed]
- Hoxha E, Harendza S, Pinnschmidt HO, et al. Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol Dial Transplant 2015;30:1862-9. [Crossref] [PubMed]
- Dong D, Fan TT, Wang YY, et al. Relationship between renal tissues phospholipase A2 receptor and its serum antibody and clinical condition and prognosis of idiopathic membranous nephropathy: a meta-analysis. BMC Nephrol 2019;20:444. [Crossref] [PubMed]
- Li W, Zhao Y. Prognostic value of phospholipase A2 receptor in primary membranous nephropathy: a systematic review and meta-analysis. Int Urol Nephrol 2019;51:1581-96. [Crossref] [PubMed]
- Morioka Y, Saiga A, Yokota Y, et al. Mouse group X secretory phospholipase A2 induces a potent release of arachidonic acid from spleen cells and acts as a ligand for the phospholipase A2 receptor. Arch Biochem Biophys 2000;381:31-42. [Crossref] [PubMed]
- Hanasaki K, Arita H. Phospholipase A2 receptor: a regulator of biological functions of secretory phospholipase A2. Prostaglandins Other Lipid Mediat 2002;68-69:71-82. [Crossref] [PubMed]
- Beck S, Beck G, Ostendorf T, et al. Upregulation of group IB secreted phospholipase A(2) and its M-type receptor in rat ANTI-THY-1 glomerulonephritis. Kidney Int 2006;70:1251-60. [Crossref] [PubMed]
- Pan Y, Wan J, Liu Y, et al. sPLA2 IB induces human podocyte apoptosis via the M-type phospholipase A2 receptor. Sci Rep 2014;4:6660. [Crossref] [PubMed]
- Jie H, Jie Y, Sun J. Mechanism and treatment of coagulation disorder in membranous nephropathy. Medical Journal of Chinese People's Liberation Army 2019;44:266-70.
- Cantarelli C, Jarque M, Angeletti A, et al. A Comprehensive Phenotypic and Functional Immune Analysis Unravels Circulating Anti-Phospholipase A2 Receptor Antibody Secreting Cells in Membranous Nephropathy Patients. Kidney Int Rep 2020;5:1764-76. [Crossref] [PubMed]
- Filippone EJ. Idiopathic membranous nephropathy and IgG4: an interesting relationship. Clin Nephrol 2014;82:7-15. [PubMed]
- Tipping PG, Kitching AR. Glomerulonephritis, Th1 and Th2: what's new? Clin Exp Immunol 2005;142:207-15. [Crossref] [PubMed]
- Shuwen L. Immune imbalance of Th9, Th2 and Treg in peripheral blood of patients with idiopathic membranous nephropathy and its correlation with disease activity. Guangxi Medical University; 2017.
- Yang G, Huang CH. secretory phospholipase A2 subfamily: structure and function. Chinese Journal of Biochemistry and Molecular Biology 2013;29:932-40.
- Fichtlscherer S, Kaszkin M, Breuer S, et al. Elevated secretory non-pancreatic type II phospholipase A2 serum activity is associated with impaired endothelial vasodilator function in patients with coronary artery disease. Clin Sci (Lond) 2004;106:511-7. [Crossref] [PubMed]
- Yedgar S, Cohen Y, Shoseyov D. Control of phospholipase A2 activities for the treatment of inflammatory conditions. Biochim Biophys Acta 2006;1761:1373-82. [Crossref] [PubMed]
- Segarra-Medrano A, Jatem-Escalante E, Carnicer-Cáceres C, et al. Evolution of antibody titre against the M-type phospholipase A2 receptor and clinical response in idiopathic membranous nephropathy patients treated with tacrolimus. Nefrologia 2014;34:491-7. [PubMed]
- Jenko-Pražnikar Z, Petan T, Pungerčar J. Ammodytoxins efficiently release arachidonic acid and induce apoptosis in a motoneuronal cell line in an enzymatic activity-dependent manner. Neurotoxicology 2013;35:91-100. [Crossref] [PubMed]
- Wong WT, Ismail M, Tohit ER, et al. Attenuation of Thrombosis by Crude Rice (Oryza sativa) Bran Policosanol Extract: Ex Vivo Platelet Aggregation and Serum Levels of Arachidonic Acid Metabolites. Evid Based Complement Alternat Med 2016;2016:7343942. [Crossref] [PubMed]
- Anthony MR. In vitro anti-platelet aggregation activity of the extracts of Protorhus longifolia. University of Zululand; 2011:140.
- May AE, Seizer P, Gawaz M. Platelets: inflammatory firebugs of vascular walls. Arterioscler Thromb Vasc Biol 2008;28:s5-10. [Crossref] [PubMed]
- Furman MI, Benoit SE, Barnard MR, et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol 1998;31:352-8. [Crossref] [PubMed]
- Nelson GJ, Schmidt PC, Bartolini G, et al. The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids 1997;32:421-5. [Crossref] [PubMed]
- Banerjee D, Mazumder S, Kumar Sinha A. Involvement of Nitric Oxide on Calcium Mobilization and Arachidonic Acid Pathway Activation during Platelet Aggregation with different aggregating agonists. Int J Biomed Sci 2016;12:25-35. [PubMed]
- Shin JH, Kwon HW, Rhee MH, et al. Inhibitory effects of thromboxane A2 generation by ginsenoside Ro due to attenuation of cytosolic phospholipase A2 phosphorylation and arachidonic acid release. J Ginseng Res 2019;43:236-41. [Crossref] [PubMed]
- Cybulsky AV. Release of arachidonic acid by complement C5b-9 complex in glomerular epithelial cells. Am J Physiol 1991;261:F427-36. [PubMed]
(English Language Editor: B. Draper)


