Omission of systematic transrectal ultrasound guided biopsy from the MRI targeted approach in men with previous negative prostate biopsy might still be premature
In the emerging field of MRI in prostate cancer (PCa) diagnosis, it has become clear that targeted biopsy with MRI guidance has additional value over systematic transrectal ultrasound-guided biopsies (TRUS-GB) alone. The MRI targeted biopsy approach increased the diagnostic yield of high-grade PCa, while concomitantly reducing the number of biopsy cores and the detection of low-grade PCa (1,2). Consequently, the question arises if in current clinical practice the TRUS-GB could be reduced or even omitted from the MRI targeted diagnostic process.
Arsov and his co-authors (3) have recently published a prospective randomized study that begins to fill this void in the literature. This study, which has a sound methodology, evaluates the additional value of TRUS-GB over MRI targeted biopsy alone, starting from the hypothesis that the approach combining targeted and systematic biopsies will be superior to targeted biopsies alone. Arsov and colleagues compare PCa detection (combined high-grade and low-grade) using MRI in-bore guided biopsy (MRI-IB-GB) or using MRI-ultrasound fusion-guided biopsy (MRI-FUS-GB) combined with systematic TRUS-GB. The study is performed in men with persistent suspicion of PCa despite having a previous negative TRUS-GB. The authors’ main conclusion is that no important increase in overall PCa detection was detected for the combined biopsy approach (39%) over MRI-targeted biopsy alone (37%), and the study was halted after interim analysis.
Despite the initial negative result, the study is a quite valuable addition to our knowledge. The design is a unique randomization of patients with positive multi-parametric prostate MRI between MRI-IB-GB versus MRI-FUS-GB + TRUS-GB, and the data merits further scrutiny as it enables us to compare MRI-FUS-GB and TRUS-GB directly (Figure 1).
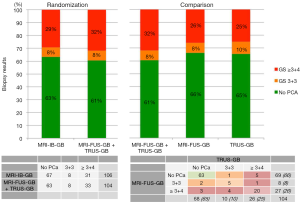
Unfortunately, the primary outcome was overall PCa detection. Increasingly, the focus in literature is shifting towards identification of only significant or high-grade PCa (Gleason score ≥3+4) as this has direct therapeutic implications, whereas low-grade PCa (Gleason score =3+3) can be managed by active surveillance and does not necessarily require treatment. Arsov and his co-authors did include high-grade PCa detection as a secondary outcome; however, no notable difference in high-grade PCa detection between the study-arms was shown (29% vs. 32%; Figure 1). Indeed, Arsov et al. showed similar results to a recent meta-analysis in which MRI-IB-GB was not superior to MRI-FUS-GB when compared to TRUS-GB for high-grade PCa detection, showing a relative sensitivity of 1.26 (95% CI, 1.08–1.46) vs. 1.29 (95% CI, 1.16–1.43), respectively (2). In the Arsov study, however, the second arm contained additional TRUS-GB, suggesting no additional value of TRUS-GB to MRI targeted biopsy alone.
In my opinion, this is only partly true. It is interesting to examine the results of the study arm MRI-FUS-GB + TRUS-GB as two separate diagnostic tests, performed in the same patient (Figure 1). The tests did not give remarkable different detection rates of high-grade PCa (26% vs. 25%) or low-grade PCa (8% vs. 10%). However, combing the tests increased high-grade PCa detection from <26% to 32%, suggesting that the combination is better than either test alone. Further analysis shows 88% concordance between the two tests in detecting high-grade PCa. The additional diagnostic yield for each test with respect to the other was 21% (7/33) for MRI-FUS-GB and 18% (6/33) for TRUS-GB. Therefore, the combined approach reduces the risk of understaging compared to MRI-FUS-GB alone, as concluded in the article. These data emphasize that combined use of MRI-FUS-GB and TRUS-GB is still warranted—indeed even recommended—as long as randomized multicenter trials comparing the single use of these biopsy techniques are pending.
Setting the results of Arsov and colleagues in the context of similar studies from the literature and applying discordance analysis yields a useful perspective. Table 1 is a summary of studies on systematic TRUS-GB and MRI-FUS-GB in patients with solely previous negative biopsies (3-8). All of these studies specified outcome regarding high-grade PCa (≥3+4). Simply summing the data indicates that MRI-FUS-GB alone is superior to TRUS-GB alone: 26% vs. 17%, respectively. More importantly, however, at first view no individual study demonstrated additional value of TRUS-GB over MRI-FUS-GB alone, with cumulative totals of 29% for the combined approach (1st column) compared to 26% to MRI-FUS-GB alone (2nd column). This is similar to the results from Arsov and colleagues. Despite this initial interpretation of the data, however, further analysis of the discordance showed an added value for TRUS-GB of 11% (22/208) for high-grade PCa detection (4th column), with the values for individual studies ranging from 7% to 18%. Such a high degree of added value for TRUS-GB cannot be neglected, when overriding the suggestion of some authors to omit TRUS-GB from the MRI targeted approach (8).

Full table
Will the urologic community accept missing these high-grade PCa and decide to abandon TRUS-GB in men with previous negative biopsies and a positive MRI? Clearly, the reduction of overdiagnosis of low-grade PCa and of the number of biopsies is a huge advantage, and therefore this approach has rapidly attracted interest among the urological community. It is becoming the new reference standard, especially in the setting when repeat biopsies are indicated. Yet the proportion of high-grade PCa missed is substantial from a clinical standpoint. The intellectual debate on this topic should be seated in the best possible interpretation of available data and engaging the right principles. We definitely need more data from prospective, observational or randomized studies together with data from hospitals other than high-volume expert centers.
In conclusion, Arsov and his co-authors have made a considerable contribution to the existing literature, bringing better insight into the added value of TRUS-GB over MRI-targeted prostate biopsy alone for the identification of high-risk PCa in men with previous negative biopsies. Further research will enrich the discussion of about omitting TRUS-GB from the MRI targeted biopsy approach in this population and enable us to reach an informed consensus before MRI targeted biopsy alone has been embraced on a large scale.
Acknowledgements
None.
Footnote
Provenance: This is a Guest Commentary commissioned by Xiongbing Zu, MD, PhD (Department of Urology, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. [Crossref] [PubMed]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Arsov C, Rabenalt R, Blondin D, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol 2015;68:713-20. [Crossref] [PubMed]
- Vourganti S, Rastinehad A, Yerram NK, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol 2012;188:2152-7. [Crossref] [PubMed]
- Durmus T, Stephan C, Grigoryev M, et al. Detection of prostate cancer by real-time MR/ultrasound fusion-guided biopsy: 3T MRI and state of the art sonography. Rofo 2013;185:428-33. [PubMed]
- Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol 2014;65:809-15. [Crossref] [PubMed]
- Mendhiratta N, Meng X, Rosenkrantz AB, et al. Prebiopsy MRI and MRI-ultrasound fusion-targeted prostate biopsy in men with previous negative biopsies: impact on repeat biopsy strategies. Urology 2015;86:1192-8. [Crossref] [PubMed]
- Salami SS, Ben-Levi E, Yaskiv O, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int 2015;115:562-70. [Crossref] [PubMed]


