Lung cancer epidemiology: contemporary and future challenges worldwide
Introduction
Every scientific paper on lung cancer epidemiology should start from a citation of Isaac Adler’s monograph published over a century ago: “Is it worthwhile to write a monograph on the subject of primary malignant tumors of the lung? In the course of the last two centuries an ever-increasing literature has accumulated around this subject. But this literature is without correlation, much of it buried in dissertations and other out-of-the-way places, and, with but a few notable exceptions, no attempt has been made to study the subject as a whole, either the pathological or the clinical aspect having been emphasized at the expense of the other, according to the special predilection of the author. On one point, however, there is nearly complete consensus of opinion, and that is that primary malignant neoplasms of the lungs are among the rarest forms of disease.” (1).
Half of a century was enough to see the lung cancer prevalence rising. Quickly the disease was linked to tobacco smoking (2). During the last 100 years this “rarest form of disease” became to be the most frequent cause of cancer death in men and in some parts of the world also in women (North America, Eastern Asia, Northern Europe, Australia and New Zealand) (3). Main reason for the epidemics has been tobacco smoke, what was proven by many historical studies both on the population (4), and biological (5) level. The cause effect relationship between tobacco smoking and lung cancer occurrence is a dose-response one (6).
Diminishing of tobacco smoking prevalence can bring tangible benefits, what was demonstrated in the theoretical model (7,8), but also in empirical studies (9,10). However, in global perspective one can observe a growing trend of tobacco consumption (11), especially in developing countries (China, India) (12,13). The discussion is open whether the modern technologies in medicine can support public health efforts to control lung cancer mortality through secondary prevention. Most important here is the harms-benefit trade-off of lung cancer screening.
Cancer data and geography
Only one sixth of the world population is covered by population-based cancer registries, and one third by death certification system (14), what limits incidence analysis and makes mortality analysis proximate. Because of high fatality of lung cancer (the overall ratio of mortality to incidence is 0.87) and the very small variability in survival in different world regions (15), mortality data closely follows incidence data (3). Therefore in our review we used mortality data from the WHO mortality database (16) and results of analyses presented in Cancer Mortality Database (14). All presented rates are age standardized (ASRW), unless indicated differently.
Presented data concern mainly WHO regions embracing: WHO Africa region (AFRO), WHO Americas region (PAHO), WHO East Mediterranean region (EMRO), WHO Europe region (EURO), WHO South-East Asia region (SEARO), WHO Western Pacific region (WPRO) (17). “More” and “less” developed regions were presented according to the Department of Economics and Social Affairs UN definition (18).
Prediction of lung cancer mortality rates and number of deaths was presented for several countries, where data were available (14). Europe was presented a little more in detail considering good data availability and some specificity of the region (for half a century the continent was divided politically and economically). In AFRO and SEAROs there is no sufficiently good quality data what made impossible to present predictions of lung cancer mortality for the following two decades (16). For SEARO there were presented predictions for number of deaths for some countries (16). African countries are represented by the African region as a whole.
How many lung cancer deaths are there?
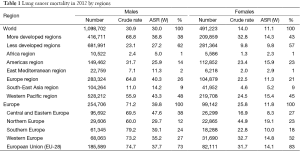
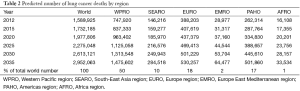
In 2012 about 1.6 million people died due to lung cancer worldwide, majority in less developed countries (63% in men, 57% in women), although mortality rates in men are almost 1.4 times higher in more developed countries, in women about 1.5 times higher (Table 1). When particular regions are taken into account the biggest number of lung cancer deaths is noted in WPRO countries (48% in men, 45% in women), EURO takes second place (26% in men, 21% in women).
Full table
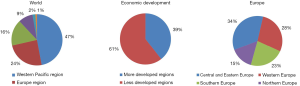
The highest lung cancer mortality is noted in WPRO countries (ASR 43.3/105 for males, 15.4/105 for females), in Europe (40.3/105 and 11.3/105 respectively) and in females in PAHO (15.5/105) (3). Almost 75% of lung cancer deaths in men and over 80% in women in Europe were noted in the European Union countries, among which majority (38% for men and 27% for women) in Central and Eastern Europe, meaning former eastern bloc countries (Figure 1). In these countries it is also observed the highest lung cancer mortality in men (47.6/105). The highest lung cancer mortality in women is observed in Northern and Western Europe, with the highest rates in Denmark (43/105), Netherlands (36/105) and UK (33/105) (3).
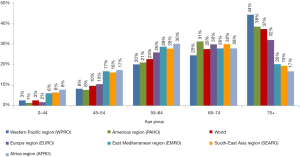
Lung cancer before the age of 44 is extremely rare in both sexes (1–8% deaths depending on the region, about 3% deaths worldwide) (Figure 2). The highest percentage of deaths in 45–64 years age group is observed in AFRO, EMRO and SEAROs (16–17%), moreover it is higher by 6–10 percentage points (pp) than in remaining regions. Similarly in middle-age (54–64 years) the percentage of deaths was highest in AFRO, EMRO and SEAROs (about 30%) whereas in European countries or Americas the percentage was lower by 10 pp. In all regions lung cancer deaths between the age 65 and 74 averaged about 30%. In countries of WPRO, EURO and PAHO over one third of lung cancer deaths occur after the age of 75, in the remaining regions it does not reach beyond 20% in this age group.
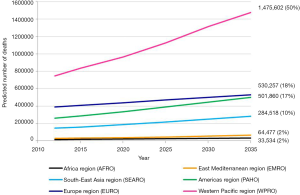
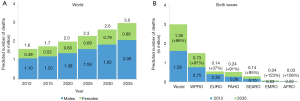
Number of lung cancer deaths worldwide will increase from 1.6 million in 2012 to 3 million in 2035. Number of lung cancer deaths will almost double in both men (1.1 million in 2012 to 2.1 million in 2035) and women (0.5 million in 2012 to 0.9 million in 2035), and number of lung cancer deaths will continue to stay twice as high as in women (Figure 3A). It is estimated that about 50% of lung cancer deaths worldwide will occur in WPRO, 20% in EURO, 17% in PAHO and 10% in SEARO. In EMRO and AFROs there will be about 1–2% of lung cancer deaths worldwide (Table 2).
Full table
It is estimated that until 2035 number of lung cancer deaths will increase globally by 86% (comparing to 2012). The increase is predicted in all regions but its scale is different. Lowest increase is predicted for Europe (37%). In few regions the number of lung cancer deaths is predicted to double (WPRO by 97%, PAHO by 91%, SEARO by 95%). In EMRO (123%) and AFRO (108%) the increase is predicted to be the highest (Figure 3B).
Increase of absolute number of lung cancer deaths is illustrated by Figure 4. WPRO will continue to have the highest number of lung cancer deaths and the gap between this region and others will widen. It seems that the main reason for that will be the increase of number of lung cancer deaths in China (compare with Figure 5). Quicker increase of number of lung cancer deaths in the Americas will bring it close to the number observed in Europe. In the remaining regions (SEARO, EMRO and AFRO) despite the rapid increase of the number of lung cancer deaths (about double in the prediction) it will not exceed 20% of the number of lung cancer deaths worldwide.
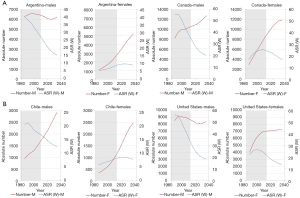
Lung cancer temporal trends in chosen PAHO countries (Argentina, Canada, Chile, USA) show two scenarios (Figure 6): (I) decline of mortality rate (ASRW) and decrease of the absolute number of lung cancer deaths—men in Argentina and the US; (II) decline of mortality rate (ASRW) and plateau or increase of the absolute number of lung cancer deaths—men and women in Canada, men in Chile, women in the US. Analogous processes were observed in Europe—the trends of lung cancer mortality in less developed European countries show some delay in the peak of mortality in relation to more developed countries (Figure 7).
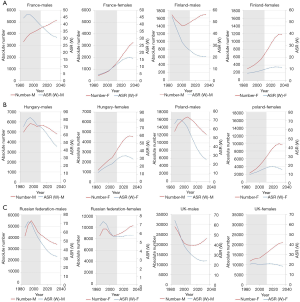
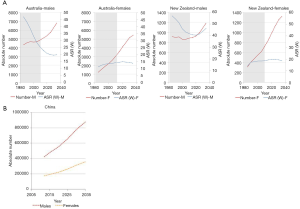
In the chosen countries of the WPRO (Australia, New Zealand), lung cancer mortality (ASRW) was declining in men and showing long-term plateau in women. These trends will hold in future. In both countries there is predicted increase of number of lung cancer deaths, significantly faster in women. Similar tendency is predicted for number of lung cancer deaths in China—until 2035 number of lung cancer deaths in men will double (up to almost 90,000) and in women it will increase by about half (up to 35,000) (Figure 5). In Japan, during the next two decades there is predicted an increase of the absolute number of lung cancer deaths in both sexes, and in the mid-20s of 21st century the numbers will level off at about 70,000 in men and 30,000 in women. In chosen countries of Eastern Mediterranean Region (Egypt, Saudi Arabia) and South-East region (India, Indonesia), similarly as for the whole region, increase of the absolute number of lung cancer deaths is predicted, especially rapid in men (the number will almost double) (3).
What is the impact of tobacco smoking?
Observed variations in lung cancer rates and trends largely follow differences in tobacco epidemic (19) because smoking accounts for about 80% of global lung cancer deaths in men and 50% of the deaths in women (20). Results of recent analysis conducted by Bilano et al. show that trends in current tobacco smoking prevalence vary between WHO regions and that several low- and middle-income countries are at risk of intensifying tobacco epidemic (11). From 2000 to 2010, majority of analysed countries experienced decreases in prevalence for both males and females. On this basis, decreases for years 2010–2025 are projected for most countries in almost all regions except AFRO for men and the Eastern Mediterranean (EMRO) for both men and women. High probabilities of decrease in current smoking prevalence are predicted for most countries in the Americas (AMRO) for both men and women. For European men, 15 (48%) high-income countries had a high probability of reduction, compared with only 4 (24%) low-income or middle-income countries, suggesting that within-region income inequalities remain an issue in tobacco control (11).
An observation of changes in smoking rates in several countries confirms that smoking patterns differ among representatives of WHO regions and can explain observed differences in tobacco-attributable cancer mortality rates and crude numbers.
In EURO region, decline in prevalence of tobacco use was observed in last decades, especially in countries of western, northern and central Europe. In Finland, France, UK and Poland prevalence of smoking has decreased by 8–43 pp among males and by 6–17 pp in female population (21-25). However, the patterns of smoking prevalence and the rate of decline differ between countries, and were higher in UK than in France (22-24). While Poland observed significant decline in last decades (25), in Hungary still 43% of adult males and 29% of females reports smoking on daily basis (23,26). In Ukraine, 45% of males and 9% of females smokes daily (27).
In EMRO countries that participated in the GATS study (Egypt and Qatar) tobacco smoking is a health problem that mainly concerns adult male population. According to GATS Egypt, in 2009 daily smokers constituted 35% of males and only 1% of females aged 15 years or older. Prevalence of adult smoking was at the same level according to data from 1998 (28). In Qatar, where GATS was conducted in year 2013, 16% of males and 2% of females smoked daily (29).
Tobacco-related statistics vary between countries in WPRO region. Decline in smoking prevalence both in male and female population was observed in both Australia and New Zealand, where current smoking rates in total population amounted respectively to 17% (in year 2011) and 24% (in year 1996) (30-32). In China, tobacco smoking rates are increasing since 1980s, particularly in male population (33). In a recent GATS study, 45% of males and 2% of females reported daily smoking (12).
Similarly as in countries of EMRO region and China, in countries of SEARO region, significant differences in smoking rates between female and male populations were observed. In countries that participated in GATS (Bangladesh, India, Indonesia, Thailand), daily smoking rates among adult females do not excess 3%. In male population, highest rates of daily smoking were observed in Indonesia and Thailand (59% and 39%, respectively). In Bangladesh 32% and in India 15% of males reported daily smoking (13,34-36).
In AMRO region prevalence of smoking differs among countries. USA and Canada observed a significant reduction in smoking rates in last decades, as current smoking is reported by 24% of males in USA and 19% in Canada. Among females those percentages amount to 18% and 15% respectively (37,38). In Central and Southern America prevalence of smoking varies between countries. In Argentina 29% of males and 16% reported daily smoking in GATS study [2013] (39), while in Mexico, respective percentages were 12% and 4% (40). Smoking rates for both sexes are substantially higher in Chile, where 44% of males and 37% of females reported current smoking (41). While a decline in smoking rates was observed in Argentina, in Chile none positive changes were identified in last decades (39,42).
Due to lack of available recent data and representative studies dedicated to tobacco use it is hard to assess the smoking prevalence in AFRO region. In 2012, GATS study has been carried out in Nigeria, where 6% of males and less than 1% (0.3%) of females reported daily smoking (43). In Algeria, daily smoking was recently reported by 25% of adults (44).
Conclusions
In many western countries, where tobacco epidemics was on the rise since the beginning of 20th century and peaked in its half (in the US, UK, Canada and Australia) or a bit later (France, Finland, Poland), the increasing trend of lung cancer incidence in men reversed or reached plateau. Despite the predicted further decrease of incidence rates, the absolute number of lung cancer deaths in men in these countries will continue to ascend, with the exception of the US, where number of lung cancer deaths is expected to level off. This increase results from demographic changes, predominantly aging of populations.
In women in developed countries the increase of lung cancer mortality rates is expected to be continued for another decade or two, and no sooner than 4th decade of 21st century one can expect the rates to level off. In these countries a rapid increase of number of deaths is expected until the plateau occurs. In women the increase of number of lung cancer deaths is caused by two factors: stable tobacco smoking prevalence and population aging. Additional contributor to the increase of lung cancer number of deaths is the fact of entering the “cancer age” by women born after the World War II, among whom tobacco smoking was particularly popular (45).
In the countries where the tobacco smoking epidemic is on the rise, it is responsible for future increase of both lung cancer mortality rate and the absolute number of deaths. The observed, almost double increase of number of deaths in WPRO and SEAROs considers both sexes and results from the predicted particularly high increase of number of deaths in countries with biggest population size (China, Korea, India, and Indonesia). In African countries, but also in Arabian Peninsula, tobacco smoking becomes more and more popular among men, what is observed in Egypt or Saudi Arabia.
Almost all lung cancer cases are caused by tobacco smoking and therefore reducing prevalence of smoking worldwide should be a basic priority in fighting the disease. Understanding epidemiology of lung cancer and its causal risk factors can provide further arguments for its prevention. Tobacco as etiologic factor for lung cancer has been indisputably proven long time ago; ionizing radiation and exposure for some workplace carcinogens are responsible for about 15–20% of lung cancer cases (46).
According to the Lopez, Collishaw and Piha model (7) it takes about 40 years for the increase in smoking rates to be fully reflected in lung cancer epidemiology statistics. Therefore changes observed in smoking prevalence in particular regions of the world will influence lung cancer picture in different ways. This increase is caused by two factors that can enhance each other: tobacco smoking prevalence (phases of the tobacco epidemic) and population ageing. The increase of percentage of older people in the population is observed in all regions of the world. In 1969–1999 the biggest increase of life expectancy was observed in East Asia and Pacific Region (over 75%), Asia and Northern Africa (ca. 40%). In the OECD countries the life expectancy increase was less pronounced (12%) (47). In PAHO (77 years), EURO and WPRO (76 years) life expectancy is still 10 or more years higher than in Africa (58 years), SEARO and EMROs (68 years) (48).
Five-year lung cancer survival rates do not exceed 15%, even in the wealthiest countries, what brings out problem of lack of effective treatment for this cancer site. However, results of treatment in early stages of disease are significantly better [in lung cancer stage I or II 5-year survival reaches 70% (49)], what makes some clinicians consider introducing low-dose CT scans for screening of high risk groups of population. One of the recent studies from the US (National Lung Screening Trial study) has shown 20% reduction of lung cancer deaths when applying such approach (50).
Despite unquestionable successes in tobacco control, especially in some more developed countries, and decline of lung cancer mortality rates in men and plateau in women, only in a few countries one can expect number of lung cancer deaths to level off. Two phenomena are responsible for the growing number of deaths worldwide (3 million deaths in 2035): aging of populations (in more developed countries) and tobacco smoking epidemics on the rise and longer life expectancy (in less developed countries).
Tobacco control is the most effective and the least expensive way to decrease number of lung cancer patients worldwide. Numerous initiatives related to lung cancer early detection has not been widely approved. Moreover, lung cancer secondary prevention cannot be considered as global strategy of controlling lung cancer, mostly due to unacceptably high costs. However, it is imaginable that countries with very high HDI might start implementation of lung cancer screening. One must remember that even then, no lung cancer screening participant can be left without being offered professional help in quitting smoking.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Adler I. Primary malignant growths of the lungs and bronchi. London: Longmans, Green, 1912.
- Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc 1950;143:329-36. [Crossref] [PubMed]
- Globocan.iarc.fr. Redirect [cited 14 January 2016]. Available online: http://globocan.iarc.fr
- Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J 1950;2:739-48. [Crossref] [PubMed]
- Boyland E, Roe FJ, Gorrod JW. Induction of pulmonary tumours in mice by mitrosonornicotine, a possible constituent of tobacco smoke. Nature 1964;202:1126. [Crossref] [PubMed]
- Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health 1978;32:303-13. [Crossref] [PubMed]
- Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control 1994;3:242-7. [Crossref]
- Didkowska J, Wojciechowska U, Koskinen HL, et al. Future lung cancer incidence in Poland and Finland based on forecasts on hypothetical changes in smoking habits. Acta Oncol 2011;50:81-7. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society, 2013.
- Peto R, Darby S, Deo H, et al. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321:323-9. [Crossref] [PubMed]
- Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990-2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 2015;385:966-76. [Crossref] [PubMed]
- World Health Organization. Global Adult Tobacco Survey (GATS) China 2010 Country Report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/chn/en/
- World Health Organization. Global Adult Tobacco Survey (GATS) India 2009-2010 Country Report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/ind/en/
- CANCERMondial [cited 15 January 2016]. Available online: http://www-dep.iarc.fr/
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- World Health Organization. WHO Mortality Database [cited 15 January 2016]. Available from: http://www.who.int/healthinfo/statistics/mortality_rawdata/en/
- World Health Organization. WHO regional offices [cited 15 January 2016]. Available online: http://www.who.int/about/regions/en/
- International migration flows to and from selected countries: the 2010 revision. [cited 15 January 2016]. Available online: http://esa.un.org/unmigration/Definition%20of%20regions.html
- Ezzati M, Henley SJ, Lopez AD, et al. Role of smoking in global and regional cancer epidemiology: current patterns and data needs. Int J Cancer 2005;116:963-71. [Crossref] [PubMed]
- Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet 2003;362:847-52. [Crossref] [PubMed]
- Official Statistics of Finland (OSF): Tobacco statistics [Internet]. Helsinki: National Institute for Health and Welfare (THL) [cited 15 January 2016]. Available online: http://www.stat.fi/til/tupk/index_en.html
- Gallus S, Lugo A, La Vecchia C, et al. Pricing Policies And Control of Tobacco in Europe (PPACTE) project: cross-national comparison of smoking prevalence in 18 European countries. Eur J Cancer Prev 2014;23:177-85. [Crossref] [PubMed]
- Mackay J, Eriksen M. The tobacco Atlas. Geneva: World Health Organization, 2002.
- Brown J, West R. Smoking prevalence in England is below 20% for the first time in 80 years. BMJ 2014;348:g1378. [Crossref] [PubMed]
- World Health Organization. Global Adult Tobacco Survey (GATS) Poland 2010 Country Report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/pol/en/
- Tombor I, Paksi B, Urbán R, et al. Epidemiology of smoking in Hungary--a representative national study. Orv Hetil 2010;151:330-7. [Crossref] [PubMed]
- World Health Organization. Global Adult Tobacco Survey (GATS) Ukraine 2010 Country Report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/ukr/en/
- World Health Organization. Global Adult Tobacco Survey (GATS) Egypt 2009 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/egypt/en/
- World Health Organization. Global Adult Tobacco Survey (GATS) Qatar 2014 country report [cited 14 January 2016]. Available from: http://www.who.int/tobacco/surveillance/survey/gats/qat/en/
- White V, Hill D, Siahpush M, et al. How has the prevalence of cigarette smoking changed among Australian adults? Trends in smoking prevalence between 1980 and 2001. Tob Control 2003;12:ii67-74. [Crossref] [PubMed]
- Wakefield MA, Coomber K, Durkin SJ, et al. Time series analysis of the impact of tobacco control policies on smoking prevalence among Australian adults, 2001–2011. Bull World Health Organ 2014;92:413-22. [Crossref] [PubMed]
- Laugesen M, Swinbern B. New Zealand's tobacco control programme 1985-1998. Tob Control 2000;9:155-62. [Crossref] [PubMed]
- Qian J, Cai M, Gao J, et al. Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data. Bull World Health Organ 2010;88:769-76. [Crossref] [PubMed]
- World Health Organization. Global Adult Tobacco Survey (GATS) Bangladesh 2009 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/bgd/en/
- World Health Organization. Global Adult Tobacco Survey (GATS) Indonesia 2011 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/idn/en/
- World Health Organization. Global Adult Tobacco Survey (GATS) Thailand 2011 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/tha/en/
- American Lung Association. Trends in tobacco use, American Lung Association Epidemiology and Statistics Unit. Research and Program Services, July 2011. Available online: http://www.lung.org/assets/documents/research/tobacco-trend-report.pdf
- Corsi DJ, Boyle MH, Lear SA, et al. Trends in smoking in Canada from 1950 to 2011: progression of the tobacco epidemic according to socioeconomic status and geography. Cancer Causes Control 2014;25:45-57. [Crossref] [PubMed]
- World Health Organization. Global Adult Tobacco Survey (GATS) Argentina 2013 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/arg/en/
- World Health Organization. Global Adult Tobacco Survey (GATS) Mexico 2009 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/mex/en/
- Pichón Riviere A, Bardach A, Caporale J, et al. Burden of tobacco-attributable diseases in Chile. Technical paper IECS N° 8. Instituto de Efectividad Clínica y Sanitaria, Buenos Aires, Argentina, 2014. Available online: www.iecs.org.ar
- Amigo H, Erazq M. Problemas asociados al tabaquismo en Chile. Rev Chil Salud Publica 2005;9:46-50.
- World Health Organization. Global Adult Tobacco Survey (GATS) Nigeria 2012 country report [cited 14 January 2016]. Available online: http://www.who.int/tobacco/surveillance/survey/gats/nga/en/
- Khattab A, Javaid A, Iraqi G, et al. Smoking habits in the Middle East and North Africa: results of the BREATHE study. Respir Med 2012;106 Suppl 2:S16-24. [Crossref] [PubMed]
- Zatonski W, Manczuk M, Sulkowska U. Closing the health gap in European Union. Warsaw: Cancer Epidemiology and Prevention Division, 2008.
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- Palacios R. The future of global ageing. Int J Epidemiol 2002;31:786-91. [Crossref] [PubMed]
- World Health Organization. Global Health Observatory [cited 15 January 2016]. Available online: http://www.who.int/gho/en/
- Guerrera F, Errico L, Evangelista A, et al. Exploring Stage I non-small-cell lung cancer: development of a prognostic model predicting 5-year survival after surgical resection†. Eur J Cardiothorac Surg 2015;47:1037-43. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]