Multiple pulmonary cavitary nodules with pyoderma gangrenosum in patient with rheumatoid arthritis
Introduction
Pyoderma gangrenosum (PG) is a rare chronic ulcerative skin disorder of unknown etiology. The most common presentation of PG is cutaneous disorder, inflammatory papule or pustule that progresses to a painful ulcer with erythematous border and purulent base. Pulmonary involvement of PG is uncommon and less than forty cases have been reported in the literature previously (1).
About 50–70% of PG cases are associated with an underlying systemic disease such as inflammatory bowel disease, hematologic diseases, connective tissue diseases or other malignancies (1). PG patients with pulmonary involvement also frequently have underlying diseases. Inflammatory bowel disease, proctitis, leukemia, multiple sclerosis, gastritis with vitamin B deficiency, monoclonal IgA-gammopathy, rheumatoid arthritis (RA), osteoarthritis, and diabetes mellitus have all been described in patients with pulmonary manifestation of PG (1). And there is only one report that describes pulmonary involvement of PG in patients with RA (2). We report a case of multiple cavitary nodules which is associated with PG in patients with RA.
Case presentation
A 66-year-old woman was admitted to the hospital in August, 2013 with aggravated cough, purulent sputum, and ulcerative skin lesion on lateral side of her left ankle. In past medical history, she was diagnosed with RA and RA associated interstitial lung disease 7 years ago. She had been treated with non-steroid anti-inflammatory drugs, hydroxychloroquine, methotrexate, low-dose systemic steroid (methylprednisolone 2 mg).
In April and July 2011, she had been admitted to our hospital due to recurrent painful ulcerative lesion on anterior side of left ankle (Figure 1), thigh and elbow and received wound debridement and skin graft. In July, 2012 she was admitted to pulmonary department with purulent sputum, cough, and left pleuritic chest pain. Her chest CT scan showed multiple cavitary consolidations in both lungs on the background of reticular opacity and honeycombing. With empirical antibiotics therapy, the cavitary nodules are slightly decreased and she discharged.
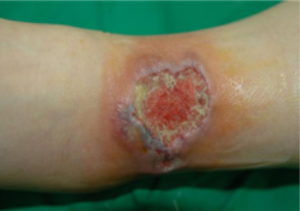
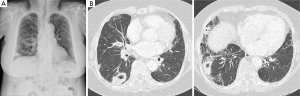
After 1 year, she was admitted again due to aggravated cough, purulent sputum and recurred ulcerative lesion on lateral side of her left ankle. On physical examination, there were a few tender, erythematous subcutaneous nodules ranging from 1 to 3 cm in diameter on her left thigh and left elbow. On her left ankle, there was about 5 cm sized ulcer with discretely elevated erythematous borders and necrotic and hemorrhagic base. Chest CT showed multiple cavitary consolidations in right middle lobe and right lower lobe (Figure 2). Although she was treated with empirical antibiotics, her symptoms and cavitary consolidations did not improved.
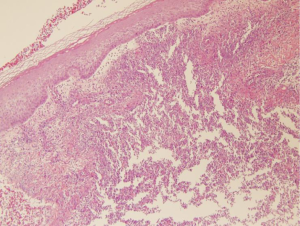
Laboratory tests revealed non-specific findings such as leukocytosis, elevated CRP. Anti-neutrophil cytoplasmic antibodies (ANCAs) are negative. Bacterial, mycobacterial and fungal cultures of blood, sputum and bronchoalveolar lavage fluid yielded no growth. Two-dimensional transthoracic echocardiography showed no valvular vegetation. Punch biopsy of erythematous nodule on thigh showed neutrophilic abscess with necrotic debris (Figure 3). It was compatible with PG. Local corticosteroids and topical tacrolimus were prescribed at her skin lesion.
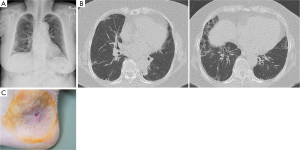
Concluding that the cavitary lung lesions are lung involvement of PG, treatment with oral prednisolone, 0.5 mg/kg/day, was initiated. After administrating steroid, her symptoms, multiple cavitary consolidations, and ulcers on skin progressively improved (Figure 4). Until now, she was continuously treated with low lose steroid maintenance therapy. The skin lesions showed wax and wane pattern improvement. But there was no relapse of multiple lung cavitary lesions on follow up.
Discussion
We report here a case of PG lung involvement in patients with RA associated interstitial lung disease. The incidence of PG is uncertain, but it is estimated to be 3–10 patients per million populations per year, occurring most commonly between third and sixth decades with a possible slight female predominance. About 50–70% of cases are associated with a variety of systemic disease, most commonly inflammatory bowel disease (3). The other systemic diseases which have been associated with PG are seropositive or seronegative arthritis, multiple myeloma, paraproteinemia, diverticulitis, leukemia and so on (4).
Visceral involvements of PG are rarely reported. They have been occurred in lungs, liver, bone, spleen, major airway and so on (1,5-7). Although the lung is the most frequent site of extracutaneous disease, pulmonary involvement of PG is also very rare and only less than forty cases have been reported in the literature previously (1). The reported pulmonary manifestations are mainly nodules with or without cavitation, but interstitial lung disease, pleural effusion were also reported (7,8).
PG with lung involvement, like PG itself, is frequently associated with systemic disease, such as inflammatory bowel disease, hematologic malignancies, tuberculous adenitis, and chickenpox and so on. There was only one case which reported PG lung involvement in patient with RA. Fukuhara et al. reported a Japanese female patient with RA who presented with PG and pulmonary aseptic abscess responding to dapsone (2).
The etiology and pathogenesis of PG are poorly understood, but neutrophil dysfunction including defects in chemotaxis and hyperreactivity has been suggested (4). In addition, many other abnormal immune responses such as compliments abnormalities, immune complex deposition and circulating factors that influence lymphocyte function have been described (8).
The most important differential diagnosis of PG lung involvement is granulomatosis with polyangiitis. Granulomatosis with polyangiitis is ANCA-associated vasculitis that involves both lungs and kidney mainly. Its clinical and histopathologic findings are similar to those of PG lung involvement. In this case, we can exclude granulomatosis with polyangiitis by the absence of nasopharyngeal and kidney involvement and negative c-ANCA.
There is no laboratory finding specific to PG, but patients often demonstrate a neutrophil leukocytosis and elevated erythrocyte sedimentation rate (4). The histopathologic findings of PG are not specific. The main feature is predominant neutrophil infiltration with tissue necrosis and inflammation around vessels. Vasculitis and signs of infection is usually absent (1).
In this case, the response to systemic steroid therapy was very good. Because there are very few controlled trials of treatment for PG, treatment has developed largely on an empirical basis (3). Although largely based on empirical basis, corticosteroid is effective in most PG cases (8-10). In addition to corticosteroid, there are some effective medications like cyclosporine, dapsone, azathioprine, infliximab etc. (4).
Prognosis of PG depends on the associated diseases (8). Although there are few data on long term outcome of PG lung involvement, there are some cases that report no relapse for several years (1). Our patient also showed no relapse for 2 years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gade M, Studstrup F, Andersen AK, et al. Pulmonary manifestations of pyoderma gangrenosum: 2 cases and a review of the literature. Respir Med 2015;109:443-50. [PubMed]
- Fukuhara K, Urano Y, Kimura S, et al. Pyoderma gangrenosum with rheumatoid arthritis and pulmonary aseptic abscess responding to treatment with dapsone. Br J Dermatol 1998;139:556-8. [PubMed]
- Ruocco E, Sangiuliano S, Gravina AG, et al. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol 2009;23:1008-17. [PubMed]
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004;43:790-800. [PubMed]
- Vadillo M, Jucgla A, Podzamczer D, et al. Pyoderma gangrenosum with liver, spleen and bone involvement in a patient with chronic myelomonocytic leukaemia. Br J Dermatol 1999;141:541-3. [PubMed]
- Kanoh S, Kobayashi H, Sato K, et al. Tracheobronchial pulmonary disease associated with pyoderma gangrenosum. Mayo Clin Proc 2009;84:555-7. [PubMed]
- Wang JL, Wang JB, Zhu YJ. Pyoderma gangrenosum with lung injury. Thorax 1999;54:953-5. [PubMed]
- Chahine B, Chenivesse C, Tillie-Leblond I, et al. Pulmonary manifestations of Pyoderma gangrenosum. Presse Med 2007;36:1395-8. [PubMed]
- Kasuga I, Yanagisawa N, Takeo C, et al. Multiple pulmonary nodules in association with pyoderma gangrenosum. Respir Med 1997;91:493-5. [PubMed]
- Krüger S, Piroth W, Amo Takyi B, et al. Multiple aseptic pulmonary nodules with central necrosis in association with pyoderma gangrenosum. Chest 2001;119:977-8. [PubMed]


