Toward precision medicine in Parkinson’s disease
The notion of precision medicine evolved over time and matured only recently. It became a hot topic not only in the medical community but also in public sphere since president Obama announced the “Precision Medicine Initiative” at the beginning of 2015. In principle, the term “precision medicine” (1) was proposed as a conceptual framework, not a new concept. Its core, in which prevention and treatment strategies are based on taking individual variability into account, has been applied in practice for centuries since the earliest classifications and diagnosis of disease and the specific therapy prescribed (2). Personalized medicine or individualized medicine, the term that has been used for the past decade, is considered as the origin of precision medicine and still widely used interchangeably. The precise meaning of these terms continues to evolve from their original conception. Personalized medicine mainly refers to identifying patients who are most likely to benefit from a specific treatment or experience side effects by defining disease biomarkers and subtypes, to improve clinical outcomes and minimizing adverse effects. Precision medicine emphasizes an innovative approach based on individual characteristics including genetic, biomarker, phenotypic, or psychosocial of each patient. Although there is similarity and overlap between the concept of personalized medicine and precision medicine, the latter term has recently become preferred among most scientists and clinicians who are applying genomics and data mining to classify individuals into subgroups with different susceptibility to a disease. What is new for the concept of “precision medicine” is the emerging approaches and technologies which enabled tailored treatments targeted to the needs of individual patients according to the precise genetic, behavioral, biomarker characteristics and bioinformatics that underpins the personalization of medical care. For example, due to the sources of phenotypic and genotypic big data analyzed by computational tools and newly available genetic editing technologies, targeted treatments are available for genetically defined subgroups of patients, enabling the provision of the right medication at the right dose for the right patient.
A multidimensional data-driven approach is about to upgrade “precision medicine” to a higher level of greater individualization in healthcare, a shift towards the treatment of individual patients rather than treating a disease, which is being driven by the convergence of the big data and OMICS revolutions. Emerging new tools make it possible to collect large amounts of digital data from different perspectives. These new sources of data, together with increasingly available molecular information from the genome and proteome etc., have created much value and interest in personalized medicine treatments based on the new phenotypic databases. Many new types of databases linked with molecular databases are now available to bolster the precision medicine concept; most of them were not readily accessible just a few years ago. In such a circumstance, patients are not only categorized by disease groups or subgroups, but also treated as individual cases based on the multi-scale data. Disease is analyzed according to genomics as well as molecular data and systems biology. The computational approaches incorporate a wide range of these personalized data sources to establish the patient’s disease data at the multidimensional levels, covering traditional sources of medical information (the history, physical examination, and laboratory panel), imaging, genomic and other OMICS analyses (such as proteomics, metabolomics, epigenetics, cell sorting, diverse cellular assays), to individualized objective phenotypic data on function and overall health status (3) by mobile health technology. It rapidly expands the scope of precision medicine and deepens our insight into it by redefining the classification of disease, interpreting this big data with important prognostic and treatment implications, and facilitating their application in clinical setting.
Thus, this review focuses on highlighting the perspectives of precision medicine in Parkinson’s disease (PD), a neurodegenerative disorder in which there is much phenotypic heterogeneity without accepted classification criteria of subtypes (4). Precision medicine in PD is expected to integrate the best evidence-based knowledge to individualize management in future health care for those with PD.
OMICS in Parkinson’s disease (PD)
OMICS includes genomics, proteomics, metabolomics and microbiomics etc., these high-throughput technologies developed rapidly during the past years, allowing us to clarify the complex pathogenesis underlying complicated biological phenomena. The multiple ‘OMICS’ profiles for each individual facilitate a better understanding of their physiological or pathological states at a specific moment and monitoring of their conditions regularly, and the integration of the OMICS profiling in precision medicine enables clinicians to offer a tailored treatment to a particular patient, including those with PD.
Tremendous progress has been made in understanding genomics of PD since the identification of the mutation in the α-synuclein (SNCA) in 1997, which encoded the major component of Lewy bodies (LBs) and Lewy neurites (LNs), the hallmarks of PD pathology (5). More than 16 loci and 11 associated genes have been identified so far (6), which improves our understanding of the pathogenesis and heterogeneity of PD. The cost of whole exome or genome sequencing reduces to $1,000 or less per case, which makes it possible to reevaluate the prospect of genetic testing (7) and its greater impact on clinic use in the era of precision medicine (8). For example, genetic testing provides key clues in diagnosing familial PD or some atypical parkinsonism (9), and helps clinicians design tailored treatment for individuals based on the prognostic predictions, such as avoiding the medication with adverse effect of cognitive impairment in those patients harboring a particular gene with greater predisposition to dementia. Furthermore, it may also help a patient make important decisions in prenatal counselling. For unaffected family members carrying the causative gene, genetic testing can be used for early diagnosis and guidance of prevention (10). In the future, the most exciting and promising studies will focus on some potential therapeutic targets, such as the reduction and clearance of α-synuclein, which might slow down or halt the disease process (11).
Microbiome, an ecological community of commensal and pathogenic microorganisms embedded on the surface of our skin and mucosal tracts, is attracting more attention in the PD field due to the advances in DNA sequencing. It is now widely recognized that the microbiome of a certain individual represents a complex fusion in innate immunity, organisms introduced in early life, diet, lifestyle, exposure to antibiotics and other environmental factors. Multiple investigations centering on microbiomes in various areas might offer a novel approach for precision medicine (12). In many cases of patients with PD, the prodromal symptoms of gastrointestinal manifestations frequently occurs before motor dysfunction appears, the correlation from the gut to brain might be caused by the dysregulation of brain-gut axis via the gut microbiota (13-15) and the misfolding of α-synuclein in enteric nervous systems (16-19). Alpha-synuclein-related neurodegeneration in the enteric nervous systems was shown to be correlated with chronic constipation and pathophysiological changes in the intestinal wall (20-22). More evidence is in favor of the hypothesis that misfolding of α-synuclein could spread from enteric nervous systems towards the central nervous systems through vagal nerve and the opposite direction of the process could happen as well (23). It was consistent with the findings that gut microbiota exert influence on brain activity, behavior and neurotransmitters or vice versa (24-27). Several studies suggested gut microbiota might affect cellular secretion of α-synuclein via regulating the activity of enteric neurons (24,28,29). Yet the relationship between gut microbiota and cellular misfolding of α-synuclein still remains unclear. The abundance of Prevotellaceae (30) and the genera Blautia, Coprococcus, and Roseburia (31) were observed to be significantly reduced in feces of PD patients. On the other hand, the motor dysfunction was improved after biopsy-proven Helicobacter pylori eradication in PD patients independent of any antiparkinsonian medication in a randomized placebo-controlled trial (32). As aforementioned, early pathology of α-synuclein was detected after applying parasympathetic innervation to the gut, which implied the association of the vagal nerve with α-synuclein–related neuropathology from the enteric nervous systems to the central nervous system (33-35). The communication between gut microbiota and the brain could be regulated by the activity of vagal afferents (36,37). Therefore, a better understanding of gut microbiota composition and the brain-gut microbiota axis interactions might offer a novel opportunity for clarifying pathophysiology of PD, making an earlier diagnosis, and providing individualized therapeutic options with anti-inflammation therapy or even vagotomy targeting in certain types of PD subgroups. Further research is needed in the future to confirm the causative correlation between gut microbiota and motor dysfunction in PD patients before utilizing antibiotic therapy to treat PD.
Molecular imaging in Parkinson’s disease (PD)
Molecular imaging allows a window into the pathophysiology of PD in vivo, as well as measuring the severity and progression. Even though it is not being considered as a key part of precision medicine in PD now, it plays a more important role in how the diagnosis is made (4). The dopamine terminal dysfunction can be demonstrated by positron emission tomography (PET) or single photon emission computed tomography (SPECT) with different tracers (38), which contribute to early and accurate diagnosis leading to appropriate medications. PET/SPECT imaging, integrated with other individual information such as genetic testing, can help the healthcare provider design tailored interventions. For instance, molecular imaging in the asymptomatic carriers with gene mutation of PD could illustrate the disease progression at its preclinical stage, thus permitting the use of neuroprotective treatments at the right time in appropriate individuals in the future.
Meanwhile, investigation of other neurotransmitter tracers such as acetylcholine, glutamate and some other pathogenesis related tracer are also needed to explore the complicated pathogenesis and progression of PD (39). For instance, neuro-inflammation ligand imaging the microglial activation might guide the individualized application of non-steroidal anti-inflammatory therapy on PD patients in the future (40). With advancement in better understanding the nature of neurodegenerative deterioration, personalized medical approach for prevention or treatment will come true eventually.
Novel tracers for imaging α-synuclein are under development and would be of particular importance as well, because abnormal deposition of α-synuclein is a pivotal pathogenesis in most cases with PD. But there is no tracer available for in vivo specifically imaging α-synuclein in human now (41-43). The visualization of α-synuclein in imaging will enable us to distinguish those PD patients without α-synuclein aggregation like those with Parkin gene mutations (44) and then exclude them from participating in immunotherapy trials targeting α-synuclein (45). Taken together, molecular imaging plays a crucial role in serving as diagnostic, prognostic, monitoring biomarkers of PD, its development will undoubtedly lead to a better integration of precision medicine in PD.
Deep brain stimulation (DBS) in Parkinson’s disease (PD)
DBS is an effective surgical procedure for the treatment of advanced PD patients. In the past 25 years, it has been performed in more than 12,000 patients across the world. The potential mechanism of DBS is to block abnormal neural signals which lead to clinical symptoms of PD via sending electrical impulses to specific brain regions. As a highly specialized and precise procedure, DBS has been confirmed to be an effective treatment among the candidate PD patients suffering from specific motor symptoms or complications. From the perspective of precision medicine, one of the key considerations in DBS is to select the most effective target area individually. As key components of cortico-basal ganglia-thalamo-cortical axis, subthalamic nucleus (STN) and the internal part of the globus pallidus (GPi) are identified to be most effective targets for treating Levodopa-responsive motor symptoms and complications.
For instance, levodopa-induced dyskinesia, a troublesome complication caused by long-term levodopa treatment, is one of the major indications for DBS surgery. Remarkably, reducing the dosage of levodopa through the STN-DBS surgery is an obvious and effective way to reduce levodopa-induced dyskinesia, meanwhile, GPi-DBS is proven to impact the basal ganglia loop and directly reduce levodopa-induced dyskinesia.
Side effects would be another major consideration when choosing the best target. Recent studies indicated side effect like cognitive decline, speech difficulty, imbalance, gait disorders, depression, declined visuomotor were more common in STN-DBS than GPi-DBS (46). So a careful preoperative assessment is vital for the favorable outcome. According to the criteria of selecting the DBS target between STN and GPi, GPi is recommended in the patients suffering from psychiatric symptoms, gait deficits, speech problem, postural instability, as well as highly advanced patients who can’t bear the risk of cognitive impairment and psychiatric/medical comorbidity by STN-DBS (47,48). On the other hand, the adverse events induced by anti-PD medication, such as levodopa-induced dyskinesia, hallucination, and aggressiveness would be decreased after receiving STN-DBS (49). Also, battery life is practical issue in the comparison of targeting STN or GPi, since much more energy is needed for GPi-DBS due to the large anatomical structure. However, the importance of this issue has been significantly lessened because of the more application of chargeable DBS pacemaker.
Another target has been used for treating PD is the ventralis intermedius (Vim) nucleus in the thalamus. Vim-DBS was reported to be able to stop the refractory tremor since 1987, but only a few patients with predominant longstanding tremor are considered to be appropriate candidates for Vim-DBS (50) because of its limitation of little improvement in akinesia and rigidity.
In advanced PD, gait and balance disorders are two of the dominant motor disabilities. Increasing bodies of evidence has indicated that low-frequency stimulation of the pedunculopontine nucleus area (PPN-DBS) can be effective for treating gait and balance disorders, on which neither STN-DBS nor GPi-DBS shows significant effect (51,52). The pilot PPN-DBS was performed in PD patients previously implanted with STN-DBS or zona-incerta-DBS in open label trials, exhibiting significant improvement in gait and balance deficits as well as other parkinsonian symptoms. Nonetheless, the efficacy of PPN-DBS was variable when it was the only target area (53,54). Interestingly, another randomized, double-blind, cross-over study suggested that the combination of PPN-DBS and dopamine replacement treatment showed significantly helpful effect on the freezing of gait (55).
Despite the well-established evidences that support DBS as an effective option for advanced PD, the feasibility is still limited by effects, side effects,, battery consumption and costs. DBS is still undergoing a revolutionary upgrading. To achieve automatic adjustment to the brain response in real time by regulating stimulation parameters, adaptive DBS was introduced in 2006 and brought about the better control of motor outcomes and marked reductions in side effects (56). Chen et al. performed an interesting experiment exhibiting that consecutive stimulation of STN may be unnecessary especially during “on” state in PD patients with motor fluctuation, instead, it may deteriorate motor functions. Since then many researches revealed that the effectiveness of adaptive DBS showed the advantages over the conventional continuous DBS, such as minimizing side effects, optimizing therapeutic efficacy, and prolonging battery life (57-59). Basically, adaptive DBS generally contains four parts, including stimulating electrodes, a physiological biomarker recorder, a controller, and a control algorithm. The physiological biomarker recorder is the key element that offers feedback signals to the controller, therefore stimulation parameters could adaptively modify. The local field potential, spike, electrocorticogram, near infrared spectroscopy, electroencephalogram, magnetoencephalography, etc., can be used as internal biomarkers to provide feedback signals. In the meantime, wearable sensors can record the motor changes of patients as an external biomarker. Taken together, they could record feedback signals to adjust stimulation parameters from the body in a real time manner (60,61). A recent trial of unilateral adaptive DBS showed that it was more effective than conventional continuous DBS in improving UPDRS performance by mediating stimulation of β activity, which referred as the local field potential recorded from STN area (57). A bilateral adaptive DBS study confirmed that both axial and limb symptom could be improved and independent bilateral sensing and stimulation could track the need for levodopa administration according to the amplitude of β activity at the corresponding electrode (58). In summary, adaptive DBS can provide a precision and individualized treatment for each patient through automatically adjusting parameters according to the real-time brain/body response (62), and a personalized option by adopting spontaneous stimulation parameters to suit the needs for individual patient (63).
Wearable sensors
The rapid advances in molecular imaging and OMICS have made it possible to customize the health care of individual PD patients, with physiological and pathological insight into the subtype and stage of the disease (3). On the other hand, the feasibility of mobile medical equipments could provide clinicians with individualized objective phenotype data on function and overall health status. Unlike the traditional clinical assessment mainly depending on subjective judgement of physicians, wearable sensor technology with the characteristics of being objective, sensitive, accurate and real-time, brings big data into the process of patient profiling. The introduction of big data becomes an important reason for the upgrading of “personalized medicine” to “precision medicine” (64), dividing PD patients into subpopulations from the aspects of precise assessment of motor function, motor subtypes classification, prognostic prediction and treatment evaluation.
Traditionally, the multiple motor symptoms in PD are assessed by clinical rating scales, including the most widely used UPDRS-III score, which consists of separate items regarding different motor symptoms with discrete grading scores from 0 to 4. Physicians utilize the UPDRS-III score to roughly evaluate the severity of the disease, however, it does not meet the demand for precision medicine.
In comparison to UPDRS-III score, wearable sensors can record and analyze motor symptoms by attaching wearable sensors on different body parts of the patients. Significant correlation was revealed between the objective measures and the clinical rating score, which showed the potential of the objective measures. Rapid alternating movement is used for the evaluating bradykinesia in clinic, which could be objectively measured by using a foam handball in subjects’ hands that was attached to angular displacement sensors (65). In terms of the postural instability evaluation, there are two currently accepted gold-standards: the laboratory measures of sway from a force-plate and the postural instability gait difficulty (PIGD) sub-score related to clinical postural instability. Mancini et al. reported an objective measure referred as “JERK”, exhibiting correlation with both the gold-standard from the force-plate and PIGD sub-score (66). In a study aiming to characterize the dynamics of arm swing coordination during walking, subjects wore forearm accelerometers during extended walking trials. Higher arm swing asymmetry was found in PD subjects and was significantly correlated with the UPDRS-III score of limbs (67).
PD is of great heterogeneity in clinical profilings, often divided into tremor dominant and PIGD motor subtypes (68). Compared to tremor dominant, the PIGD patients were likely to suffer from a more rapid decline and higher possibility of developing dementia (69). Therefore, it was relevant to distinguish between the two motor subtypes and to characterize the heterogeneity, as an important reference to the prognostic prediction and treatment decision-making. The instrumented time up and go (iTUG) was a technological revolution of the traditional time up and go (TUG) test (70). In the study of investigating the feasibility of iTUG in differentiating the tremor dominant and PIGD subtypes, significant difference was found between the tremor dominant and PIGD groups in the objective measures of walking duration, numbers of steps, turn-to-walk amplitudes, etc. In addition, imbalance was one of the major different characteristics between the two motor subtypes, which could be tested using a single inertial measurement unit (71). In Rocchi’s study, several objective postural measures were selected as the best to discriminate between the tremor dominant group and PIGD group (72).
Freezing of gait and falls are the major reasons of secondary injury in PD patients. Prediction of the risks regarding freezing of gait and fall will contribute to tailored individual health care. The acceleration measures derived by body-fixed sensors were significantly different in fallers and non-fallers, which were correlated with previously validated measures of fall risk (73). Furthermore, therapeutic equipments based on wearable sensors and rhythmic auditory systems have been developed to detect freezing of gait and help patients resume walking on time simultaneously (74).
The long-term use of levodopa replacement treatment in PD would lead to complications of disabling fluctuations and levodopa-induced dyskinesia. Using a wrist-worn sensor, the measurement system could not only detect the presence of levodopa-induced dyskinesia with sensitivity of 0.73 and specificity of 1.00 (75), but also demonstrate a correlation coefficient of 0.81 between clinician and model scores (76). The study under real-life conditions indicated that the proposed method was highly efficient for levodopa-induced dyskinesia assessment, including detection of levodopa-induced dyskinesia symptoms and classification of their severity (77). Meanwhile, long-time monitoring of the principal motor symptoms (e.g., gait and tremor) of PD could also reflect the medication effects indirectly by assessing the motor fluctuation during daytime (78-80).
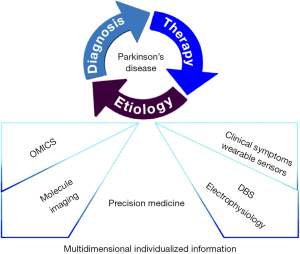
In summary, precision medicine in PD is put forward initiatively in the context of rapid development in molecular biology and computational approaches, making deeper understanding towards the physiological and pathological mechanisms of the disease. Individualized evaluation and tailored therapeutic strategies will be built based on multi-dimensional information about OMICS, molecular imaging, DBS surgery and wearable sensors (Figure 1). It is reasonable to expect that the idea of precision medicine will become a promising and feasible strategy of providing more professional benefit to PD patients in the near future.
Acknowledgements
Funding: This work was supported by grants from the National Foundation of Natural Science of China (No. 81071018, No. 81371413, No. 81571232), a key project from the Science and Technology Commission of Shanghai Municipality (13JC1401103), and a project of the Shanghai Municipal Commission of Health (XBR2013088).
Footnote
Provenance: This is a Guest Review commissioned by Guest Editor and Associate Editor-in-Chief Prof. Jin-Tai Yu (Department of Neurology, University of California, San Francisco, California, USA) and Guest Editor Dr. Shi-Wen Wu (Department of Neurology, General Hospital of Chinese Armed Police Forces, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229-34. [PubMed]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793-5. [PubMed]
- Mohler J, Najafi B, Fain M, et al. Precision Medicine: A Wider Definition. J Am Geriatr Soc 2015;63:1971-2. [PubMed]
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591-601. [PubMed]
- Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 1997;276:2045-7. [PubMed]
- Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev 2011;91:1161-218. [PubMed]
- Hayden EC. Technology: The $1,000 genome. Nature 2014;507:294-5. [PubMed]
- Lander ES. Cutting the Gordian helix--regulating genomic testing in the era of precision medicine. N Engl J Med 2015;372:1185-6. [PubMed]
- Stamelou M, Quinn NP, Bhatia KP. "Atypical" atypical parkinsonism: new genetic conditions presenting with features of progressive supranuclear palsy, corticobasal degeneration, or multiple system atrophy-a diagnostic guide. Mov Disord 2013;28:1184-99. [PubMed]
- Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson's disease. Eur J Neurol 2013;20:16-34. [PubMed]
- Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson's disease: progress and therapeutic implications. Mov Disord 2013;28:14-23. [PubMed]
- Blaser MJ. The microbiome revolution. J Clin Invest 2014;124:4162-5. [PubMed]
- Braak H, de Vos RA, Bohl J, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67-72. [PubMed]
- Lebouvier T, Chaumette T, Paillusson S, et al. The second brain and Parkinson's disease. Eur J Neurosci 2009;30:735-41. [PubMed]
- Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 2008;23:1065-75. [PubMed]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306-14. [PubMed]
- Borre YE, Moloney RD, Clarke G, et al. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol 2014;817:373-403. [PubMed]
- Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015;125:926-38. [PubMed]
- Shannon KM, Keshavarzian A, Dodiya HB, et al. Is alpha-synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord 2012;27:716-9. [PubMed]
- Forsyth CB, Shannon KM, Kordower JH, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One 2011;6:e28032. [PubMed]
- Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson's disease. Neurobiol Dis 2013;50:42-8. [PubMed]
- Lebouvier T, Neunlist M, Bruley des Varannes S, et al. Colonic biopsies to assess the neuropathology of Parkinson's disease and its relationship with symptoms. PLoS One 2010;5:e12728. [PubMed]
- Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 2007;33:599-614. [PubMed]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701-12. [PubMed]
- Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394-401, 1401.e1-4.
- Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 2011;108:3047-52. [PubMed]
- Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011;141:599-609, 609.e1-3.
- Paillusson S, Clairembault T, Biraud M, et al. Activity-dependent secretion of alpha-synuclein by enteric neurons. J Neurochem 2013;125:512-7. [PubMed]
- Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol Life Sci 2013;70:55-69. [PubMed]
- Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015;30:350-8. [PubMed]
- Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord 2015;30:1351-60. [PubMed]
- Dobbs SM, Dobbs RJ, Weller C, et al. Peripheral aetiopathogenic drivers and mediators of Parkinson's disease and co-morbidities: role of gastrointestinal microbiota. J Neurovirol 2015. [Epub ahead of print]. [PubMed]
- Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 2003;24:197-211. [PubMed]
- Bloch A, Probst A, Bissig H, et al. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 2006;32:284-95. [PubMed]
- Ulusoy A, Rusconi R, Pérez-Revuelta BI, et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol Med 2013;5:1051-9. [PubMed]
- Perez-Burgos A, Wang B, Mao YK, et al. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am J Physiol Gastrointest Liver Physiol 2013;304:G211-20. [PubMed]
- Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050-5. [PubMed]
- Cummings JL, Henchcliffe C, Schaier S, et al. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain 2011;134:3146-66. [PubMed]
- Weingarten CP, Sundman MH, Hickey P, et al. Neuroimaging of Parkinson's disease: Expanding views. Neurosci Biobehav Rev 2015;59:16-52. [PubMed]
- Stoessl AJ, Martin WW, McKeown MJ, et al. Advances in imaging in Parkinson's disease. Lancet Neurol 2011;10:987-1001. [PubMed]
- Bagchi DP, Yu L, Perlmutter JS, et al. Binding of the radioligand SIL23 to α-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a Parkinson disease imaging agent. PLoS One 2013;8:e55031. [PubMed]
- Zhang X, Jin H, Padakanti PK, et al. Radiosynthesis and in Vivo Evaluation of Two PET Radioligands for Imaging α-Synuclein. Appl Sci (Basel) 2014;4:66-78. [PubMed]
- Vernon AC, Ballard C, Modo M. Neuroimaging for Lewy body disease: is the in vivo molecular imaging of α-synuclein neuropathology required and feasible? Brain Res Rev 2010;65:28-55. [PubMed]
- Lindström V, Ihse E, Fagerqvist T, et al. Immunotherapy targeting α-synuclein, with relevance for future treatment of Parkinson's disease and other Lewy body disorders. Immunotherapy 2014;6:141-53. [PubMed]
- Dehay B, Bourdenx M, Gorry P, et al. Targeting α-synuclein for treatment of Parkinson's disease: mechanistic and therapeutic considerations. Lancet Neurol 2015;14:855-66. [PubMed]
- Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain 2005;128:2240-9. [PubMed]
- Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077-91. [PubMed]
- Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 2007;318:1309-12. [PubMed]
- Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson's disease: an open-label randomised controlled trial. Lancet Neurol 2012;11:140-9. [PubMed]
- Benabid AL, Pollak P, Louveau A, et al. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 1987;50:344-6. [PubMed]
- Welter ML, Houeto JL, Tezenas du Montcel S, et al. Clinical predictive factors of subthalamic stimulation in Parkinson's disease. Brain 2002;125:575-83. [PubMed]
- Bonnet AM, Loria Y, Saint-Hilaire MH, et al. Does long-term aggravation of Parkinson's disease result from nondopaminergic lesions? Neurology 1987;37:1539-42. [PubMed]
- Moro E, Hamani C, Poon YY, et al. Unilateral pedunculopontine stimulation improves falls in Parkinson's disease. Brain 2010;133:215-24. [PubMed]
- Thevathasan W, Coyne TJ, Hyam JA, et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 2011;69:1248-53; discussion 1254. [PubMed]
- Welter ML, Demain A, Ewenczyk C, et al. PPNa-DBS for gait and balance disorders in Parkinson's disease: a double-blind, randomised study. J Neurol 2015;262:1515-25. [PubMed]
- Chen CC, Brücke C, Kempf F, et al. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr Biol 2006;16:R952-3. [PubMed]
- Rosin B, Slovik M, Mitelman R, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 2011;72:370-84. [PubMed]
- Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson's disease. J Neurol Neurosurg Psychiatry 2015. [Epub ahead of print]. [PubMed]
- Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013;74:449-57. [PubMed]
- Pham J, Cabrera SM, Sanchis-Segura C, et al. Automated scoring of fear-related behavior using EthoVision software. J Neurosci Methods 2009;178:323-6. [PubMed]
- Patel S, Hester T, Hughes R, et al. Using Wearable Sensors to Enhance DBS Parameter Adjustment for Parkinson's Disease Patients Through Measures of Motor Response. Cambridge, MA: Medical Devices and Biosensors, 2006. 3rd IEEE/EMBS International Summer School on, 2006:141-4.
- Castrioto A, Moro E. New targets for deep brain stimulation treatment of Parkinson's disease. Expert Rev Neurother 2013;13:1319-28. [PubMed]
- Eberle W, Penders J, Yazicioglu RF. Closing the loop for Deep Brain Stimulation implants enables personalized healthcare for Parkinson's disease patients. Conf Proc IEEE Eng Med Biol Soc 2011;2011:1556-8.
- Klonoff DC. Precision medicine for managing diabetes. J Diabetes Sci Technol 2015;9:3-7. [PubMed]
- Daneault JF, Carignan B, Sadikot AF, et al. Are quantitative and clinical measures of bradykinesia related in advanced Parkinson's disease? J Neurosci Methods 2013;219:220-3. [PubMed]
- Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil 2012;9:59. [PubMed]
- Huang X, Mahoney JM, Lewis MM, et al. Both coordination and symmetry of arm swing are reduced in Parkinson's disease. Gait Posture 2012;35:373-7. [PubMed]
- Marras C, Lang A. Parkinson's disease subtypes: lost in translation? J Neurol Neurosurg Psychiatry 2013;84:409-15. [PubMed]
- Burn DJ, Rowan EN, Allan LM, et al. Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006;77:585-9. [PubMed]
- Salarian A, Horak FB, Zampieri C, et al. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng 2010;18:303-10. [PubMed]
- Herman T, Weiss A, Brozgol M, et al. Identifying axial and cognitive correlates in patients with Parkinson's disease motor subtype using the instrumented Timed Up and Go. Exp Brain Res 2014;232:713-21. [PubMed]
- Rocchi L, Palmerini L, Weiss A, et al. Balance testing with inertial sensors in patients with Parkinson's disease: assessment of motor subtypes. IEEE Trans Neural Syst Rehabil Eng 2014;22:1064-71. [PubMed]
- Weiss A, Herman T, Giladi N, et al. Objective assessment of fall risk in Parkinson's disease using a body-fixed sensor worn for 3 days. PLoS One 2014;9:e96675. [PubMed]
- Bächlin M, Plotnik M, Roggen D, et al. Wearable assistant for Parkinson's disease patients with the freezing of gait symptom. IEEE Trans Inf Technol Biomed 2010;14:436-46. [PubMed]
- Mera TO, Burack MA, Giuffrida JP. Quantitative assessment of levodopa-induced dyskinesia using automated motion sensing technology. Conf Proc IEEE Eng Med Biol Soc 2012;2012:154-7.
- Mera TO, Burack MA, Giuffrida JP. Objective motion sensor assessment highly correlated with scores of global levodopa-induced dyskinesia in Parkinson's disease. J Parkinsons Dis 2013;3:399-407. [PubMed]
- Tsipouras MG, Tzallas AT, Rigas G, et al. An automated methodology for levodopa-induced dyskinesia: assessment based on gyroscope and accelerometer signals. Artif Intell Med 2012;55:127-35. [PubMed]
- Cancela J, Pastorino M, Arredondo MT, et al. Gait assessment in Parkinson's disease patients through a network of wearable accelerometers in unsupervised environments. Conf Proc IEEE Eng Med Biol Soc 2011;2011:2233-6.
- Mitoma H, Yoneyama M, Orimo S. 24-hour recording of parkinsonian gait using a portable gait rhythmogram. Intern Med 2010;49:2401-8. [PubMed]
- Mera TO, Heldman DA, Espay AJ, et al. Feasibility of home-based automated Parkinson's disease motor assessment. J Neurosci Methods 2012;203:152-6. [PubMed]


