Robotic adrenalectomy for sigmoid colon cancer oligometastasis
Introduction
Colorectal cancer is the third most common malignancy affecting males. Up to 20% of the patients reveal distant metastasis at presentation, with most common sites being liver, lung, and peritoneum in decreasing order of frequency. Literature reveals reasonably good long-term outcomes for colorectal cancers with metastasis to liver and lung. However, presence of metastasis to the adrenal gland is regarded as an indicator of widespread disease, and, hence, most of these patients are treated with palliative intent. We are reporting a case of isolated metastasis to the adrenal gland treated with curative intent.
Case presentation
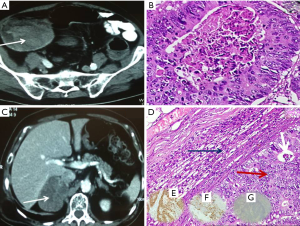
An 83-year-old man with diabetes presented to us with history of rectal bleeding of recent onset. On colonoscopy, there was a growth starting at 25 cm from the anal verge, biopsy of which confirmed it as adenocarcinoma. Contrast-enhanced computed tomography (CECT) of the pelvis and abdomen revealed thickening of sigmoid colon with well-maintained fat planes all around and no evidence of distant metastasis (Figure 1A). Carcinoembryonic antigen (CEA) level was 2.2 ng/mL. Patient underwent sigmoid colectomy and had uncomplicated postoperative recovery. Final histopathological examination revealed moderately differentiated adenocarcinoma, pathological stage pT3N0 (Figure 1B). In view of his age, patient was kept under observation, and adjuvant therapy was deferred after discussion in the multidisciplinary clinic. Nine months after the index surgery, on routine follow-up, CEA was found to be raised (4.46 ng/mL). On further investigation with ultrasound abdomen, a right adrenal mass of size 7.5 cm × 7 cm was found. [F]18-fluorodeoxyglucose positron emission tomography (18F-FDG PET) CT confirmed right adrenal metastasis with no other sites of disease (Figure 1C). Patient underwent right robotic adrenalectomy and final histopathology confirmed the adrenal lesion as metastatic deposits from sigmoid carcinoma. (On immunohistochemistry, it was CK7 negative, CK20, and CDX2 positive Figure 1D-G). In view of recurrent lesion with a short disease-free interval, patient received 4 cycles of adjuvant chemotherapy comprising 5 fluorouracil and leucovorin according to Rosewell Park regimen. At the last follow-up, patient was asymptomatic with normal CEA levels.
Discussion
In general, adrenal glands are the fourth most common site of metastasis after lung, liver, and bone (1). Most common tumors that metastasize to adrenal are lung, colorectal, and renal cancers (2). In autopsy series, incidence of adrenal metastasis has been reported to be between 1.9% and 17.4% (3). With widespread use of cross-sectional imaging, adrenal metastasis is more frequently detected than in the past. Isolated adrenal metastasis from colorectal cancer is rare with reported incidence in autopsy series from 3.1% to 14.4% (4). The potential routes of adrenal metastasis from colorectal cancer include systemic venous, portal venous, lymphatic, and hematogenous via lungs (5).
About 20% of patients with colorectal cancers show distant metastasis at the initial diagnosis (6). In addition, among those patients who undergo curative resection of the primary tumor, one-third develops recurrent disease. Most common sites of metastasis are liver, lung, ovary, and peritoneum in the decreasing order of frequency. Although systemic therapy forms the backbone of management of metastatic colorectal cancer, surgical resection in selected patients leads to improved survival. Conventionally, only patients with limited hepatic or pulmonary metastasis have been the candidates for surgical resection. Reported 5-year overall survival rate for resectable liver metastasis is about 37–58% (7). Similarly, although less well defined than liver metastasis, complete resection of lung metastasis is associated with prolonged survival in selected patients (8). In view of these improved results, the indications for surgical resection in metastatic setting have been expanded to other sites. However, such cases are rare, and, hence, only limited survival data are available.
Early detection of adrenal metastasis is of paramount importance. In this case, CEA had doubled from the previous levels and that prompted us to investigate further. Prior studies have shown that CEA is an important tool in differentiating colorectal metastasis from other pathologies in cases of adrenal incidentalomas (9). Although CECT abdomen is a cost-effective means for identifying the adrenal lesion, it cannot differentiate between primary and secondary lesions. 18F-FDG PET-CT is useful to differentiate benign from malignant adrenal lesions, with accuracy ranging from 92% to 100% (10). Fine-needle aspiration cytology (FNAC) is the best means to confirm the diagnosis of metastasis. However, it is associated with significant risk of pneumothorax and retroperitoneal hemorrhage. In this case, FNAC was not considered essential in view of the characteristic findings on PET-CT.
There is no consensus on the management of patients with isolated adrenal metastasis. Surgical resection remains controversial although a median survival of 32 months was found in the largest reported case series (11). It has been postulated that surgical resection should be offered when the adrenal metastasis develops more than 6 months after the treatment of the primary tumor (12). Minimally invasive adrenalectomy is rapidly replacing open adrenalectomy for benign lesions. However, for the metastatic lesions and potentially malignant lesions, role of minimally invasive surgery is still considered controversial (13). Role of adjuvant therapy after resection of the adrenal metastasis is not well defined in view of the rarity of these cases. In this case, robotic adrenalectomy was performed as:
- Metastasis was isolated to the adrenal gland.
- Metastasis developed more than 6 months after the treatment of sigmoid carcinoma.
- Patient was an octogenarian, and, hence, minimally invasive approach was preferable over open approach.
Conclusions
Isolated adrenal metastasis from the colorectal cancer is rare. Early detection is the key for successful management. Selected cases can be treated surgically with good results. As more cases get reported, it may be possible to draw more definite conclusions regarding its management and long-term prognosis.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95-101. [PubMed]
- Moreno P, de la Quintana Basarrate A, Musholt TJ, et al. Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery 2013;154:1215-22; discussion 1222-3. [PubMed]
- Tirziu R, Cretu O. Solitary adrenal metastasis from colorectal cancer: report of a case. J Exper Med Surg Res 2010;27:225-9.
- Glomset DA. The incidence of metastasis of malignant tumors to the adrenals. Am J Cancer 1938;32:57-61.
- Katayama A, Mafune K, Makuuchi M. Adrenalectomy for solitary adrenal metastasis from colorectal carcinoma. Jpn J Clin Oncol 2000;30:414-6. [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438-47; discussion 447-50. [PubMed]
- Yedibela S, Klein P, Feuchter K, et al. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol 2006;13:1538-44. [PubMed]
- Porcaro AB, Novella G, Ficarra V, et al. Adrenal incidentalomas: surgical treatment in 28 patients and update of the literature. Int Urol Nephrol 2001;32:295-302. [PubMed]
- Chong S, Lee KS, Kim HY, et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics 2006;26:1811-24; discussion 1824-6.
- Mourra N, Hoeffel C, Duvillard P, et al. Adrenalectomy for clinically isolated metastasis from colorectal carcinoma: report of eight cases. Dis Colon Rectum 2008;51:1846-9. [PubMed]
- Kim SH, Brennan MF, Russo P, et al. The role of surgery in the treatment of clinically isolated adrenal metastasis. Cancer 1998;82:389-94. [PubMed]
- Zografos GN, Markou A, Ageli C, et al. Laparoscopic surgery for adrenal tumors. A retrospective analysis. Hormones (Athens) 2006;5:52-6. [PubMed]


