Niemann-Pick disease treatment: a systematic review of clinical trials
Introduction
Niemann-Pick (NP) disease is caused by an abnormality in lysosomes, which are unable to degrade macromolecules; as a result, the latter accumulate inside these organelles to form cellular inclusions (1-3). NP disease encompasses a set of autosomal recessive hereditary abnormalities (1) characterized by the accumulation of lipids, mostly sphingomyelin and cholesterol, in different organs such as spleen or liver (1,4,5).
In 1961, Crocker classified the disease into 4 types depending on the organs affected and the age of symptoms’ onset: NP type A (NPA), B (NPB), C (NPC) and D (NPD) (1,6,7). In 1966, Brady et al. showed that the tissues of patients with NPA were deficient in acid sphingomyelinase (ASM) (8,9), a lysosomal enzyme whose function is to degrade sphingomyelin (9). Such deficiency also occurs in NPB, but not in NPC or NPD (7,9), with the latter being caused by a defect in the transport of intracellular cholesterol (7). Thus, the 4 disease types can be grouped into 2 main categories (1,4): (I) NPA and NPB; and (II) NPC and NPD.
In NPA and NPB, ASM deficiency results in the accumulation of sphingomyelin in different organs (1) and is caused by mutations in SMPD1 gene (11p15.1-15.4). Common manifestations of both disease types are hepatosplenomegaly and appearance of cherry-red spots in the retina (1,5) whereas neurodegeneration is only manifest in patients with NPA (4,10). NPC and NPD are characterized by a defect in the intracellular transport of low-density lipoprotein (LDL), which causes the accumulation of free (un-esterified) cholesterol and glycosphingolipids in multiple organs and tissues (4,10-13). NPC is caused by mutations in NPC1 (18q11.2) and NPC2 genes (14q24.3) in 95 and 5% of cases, respectively (4,14-16), with these two genes encoding proteins of intracellular lipid transport (12,17). NPD is caused by mutations in NPC1 and can be considered as a variant of NPC (4,14). Although NPC symptoms are variable and can occur at any age (14), when they start in early life the clinical presentation is characterized by more manifest, faster neuronal degeneration (13,14,18). In general, the most common symptoms of NPC include hepatosplenomegaly and neurologic deterioration with ataxia, motor pathologies and horizontal saccadic eye movements (HSEM) (11,14,17,19-22).
Until some years ago, the treatment for NP disease was based on different drugs such as anti-epileptics, anticholinergic or antidepressants to alleviate symptoms, i.e., tremor, dystonia or seizures (19). Miglustat (Zavesca®, Brazaves®), a small iminosugar molecule that reversibly inhibits glycosphingolipid synthesis (23) is currently available in Europe, Canada and Japan; this compound is able to delay neurological manifestations in patients with NP, type 1 Gaucher disease (GD1) or GM2 gangliosidosis type Sandhoff (13,19,24-26). In the NPA and NPB types, current research focuses on hematopoietic cell transplantation and enzyme replacement (5,10).
At present, there is no cure for NP disease and potential therapies should be addressed. A main goal is to develop treatments in order to minimize both general symptoms and neurodegeneration. For this purpose, several clinical trials have been published to assess the effects, advantages and disadvantages of recently discovered treatments for NP disease. Thus, the aim of this systematic review was to analyse all the clinical trials available in the literature assessing potential treatments for NP disease and to compare, when possible, the effectiveness of the different treatments.
Methods
Study selection
A search was conducted in ScienceDirect and PubMed to identify all the clinical trials available for the treatment of NP disease. The main search term was “Niemann Pick” but additional searches were added such as “Niemann Pick AND medication”, “Niemann Pick AND clinical trial”, and “Niemann Pick AND treatment”. During the search, there were no restrictions based on language or year of publication.
Data extraction
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations (the PRISMA statement) (27). The data extracted from the clinical trials were: article’s first author, participants’ age, total number of subjects, NP type and genetic mutation, number of participants in each group (experimental or control), weight, height and body mass index (BMI), type of treatment, as well as the daily dose, adverse effects, disease signs and symptoms, time and type of diagnosis, blood levels of total cholesterol (TC) (before and after treatment) and changes in the course of the disease.
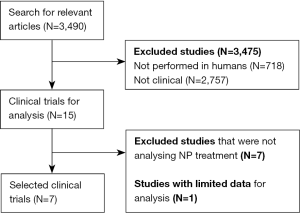
A total of 3,490 potential articles were identified, but only 15 of them were clinical trials performed in humans. Among them, 7 were excluded with reasons (Figure 1).
Results
General characteristics of clinical trials
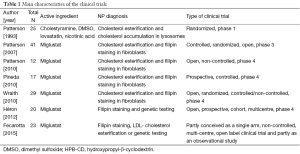
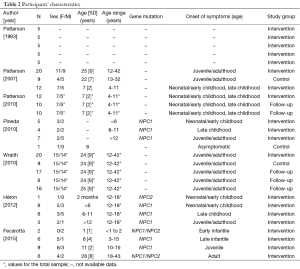
Seven clinical trials with data from a total of 167 participants were included in the present systematic review (Tables 1,2). All of them focused on NPC. Only 3 trials had a control group (12,13,24), there was one phase 1 (28) and one phase 3 trial (24), and 71% of trials were phase 4 (12,13,17,29,30). Participants’ age was not specified in two trials (12,28), and there was considerable variability among studies in patients’ age (ranging from less than 1 year to more than 40 years), as well as in total sample size (n=12 to 41). In most study cohorts the majority of patients were women. Finally, the commonest disease diagnostic techniques were demonstration of filipin staining or LDL-cholesterol esterification to evidence intracellular accumulation of cholesterol in patients’ skin fibroblasts, followed by genetic testing.
Full table
Full table
Miglustat
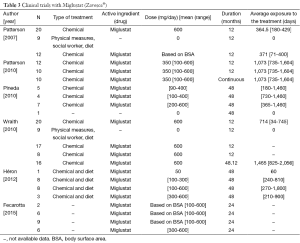
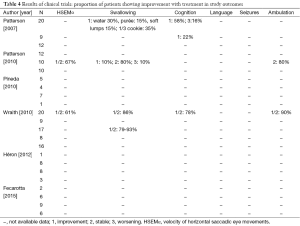
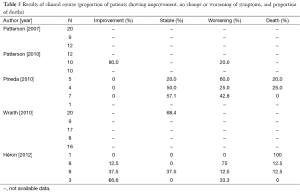
Six of the clinical trials used miglustat (Table 3). For those aged >12 years, the dose was 200 mg thrice a day, while for children the dose was calculated from the body surface area (BSA) using the following equation (29): dose (mg) = [(BSA, m2)/1.8]×200. Treatment duration varied from 12 to 60 months and 2 studies (13,29) allowed participants to continue with the intervention (Table 3). Outcome data on swallowing, language, manipulation, seizures, cognition, ocular movements and ambulation were limited, or not shown in two of the studies (12,17) (Table 4). Five of 6 trials divided their final results in subgroups depending on the disease course during the treatment (Table 5).
Full table
Full table
Full table
In the study by Patterson et al. (24) (n=29 patients aged >12 years), the proportion of participants showing improvement with the drug was 30% for water intake, 15% for swallowing puree, 15% for soft food intake and 35% for swallowing a third part of a cookie. Yet 58% of participants in the intervention group showed no improvement in cognition parameters while 16% experienced deterioration. In the control group (n=9), only cognition data were available, with 22% of participants showing an improvement.
Pineda et al. reported no numerical results (12). However, by observing the data shown in their published Figures, it can be inferred that: (I) 20% of early onset patients (<6 years) showed a stable disease course, while 60% deteriorated and 20% died during the trial; (II) 50% of participants aged 6-11 years remained stable, 25% of them died and 25% worsened; and (III) 57.1% of juvenile/adult onset patients remained stable, while 42.8% suffered clinical deterioration. One of the participants in this trial did not have symptoms and his data were included as a control.
Patterson et al. (29) reported that: (I) 67% of 10 patients showed an improvement, or at least stagnation, in HSEM velocity after 24 months of treatment; (II) 10% of the subjects improved swallowing (vs. 10% showing deterioration and 80% remaining stable); and (III) 80% of the patients maintained a stable ambulation. Patients who did not show a worsening in swallowing and ambulation parameters or those who only worsened in HSEM velocity by 20% or less, were considered to be overall stable patients. Using this criterion, it was concluded that 80% of participants remained stable while 20% worsened.
The trial carried out by Wraith et al. (13) showed results from an experimental (n=20) and follow-up group (n=17) after 24 months of treatment in only one outcome, swallowing, and without a parallel control group receiving no treatment. After 12 months of treatment with miglustat HSEM velocity improved or remained stable in 61% of patients, whereas swallowing remained stable or improved in 78.9%. The treatment effects improved after 24 months, when 79-93% of the subjects were stabilized or showed an improvement in HSEM velocity. An 89% of the patients improved or maintained stable ambulation capacity whereas 77.8% stabilized or suffered an improvement in cognition. Depending on the evolution of the participants in the different parameters, this trial determined the amount of patients who showed a stabilisation during treatment as follows: (I) increase or decrease within ±20% in HSEM; (II) no changes in swallowing; (III) ambulation did not change or changed only 1 point; and (IV) cognition varied within 2 points. Using the abovementioned criteria, this study found that 68.4% of participants (n=20) remained stable after 12 months of treatment.
The trial of Heron et al. (17) showed no specific data for each clinical parameter, but they reported the individual results of each participant and an overall favourable response to the treatment. Trial results were divided according to the onset of NP. The only patient with perinatal NP (2 months) died during the trial. Among early childhood (<6 years) onset patients, 12.5% remained stable, 75% deteriorated and 12.5% died. Among late childhood (6-11 years) patients, 37.5% remained stable, 37.5% improved, 12.5% experienced deterioration and 12.5% died during the study. Finally, 66% of patients with juvenile (>12 years) NP remained stable while 33.3% suffered deterioration.
Finally, the trial by Fecarotta et al. (30) reported that most patients showed stabilization or improvement in neurological involvement, developmental delay or cognitive impairment, gait abnormalities, dystonia, dysmetria or dysarthria. Moreover, 20 patients showed stabilization or improvement in the ability to swallow water (65%), purée (58%), small amounts of pasta (60%) and biscuit (55%). Lastly, the severity score of the symptoms improved in 57% of patients. However, during the treatment epistaxis and thrombocytopenia, insomnia, leukopenia, behavioural problems, extrapyramidal symptoms, tremors, hypertransaminasemia, and especially weight loss or diarrhea, were detected in some patients.
Of the selected clinical trials, all but one reported common disease symptoms at baseline, i.e., ataxia, speech difficulties, hepatomegaly, splenomegaly, cataplexy and vertical supranuclear gaze palsy (VSGP). All of them reported the potential adverse effects of miglustat, i.e., diarrhea (present in 83.3% of patients), flatulence, weight loss and tremor (66.6%), and headache, fatigue, nasopharyngitis, dysphagia, vomiting, cough, falls and sinusitis (50%).
Other treatments
Patterson et al. (28) assessed the effects of dimethyl sulfoxide (DMSO), nicotinic acid, lovastatin, cholestyramine, and combinations thereof on cholesterolemia but did not report neurological outcomes. The treatment effects on TC varied depending on the drug combinations and overall improved with the number of drugs: (I) in the first group, treated with DMSO only, TC increased by 7.4%; in those receiving a combination of DMSO and lovastatin, TC decreased by −20.6%; (II) those treated with DMSO, lovastatin and cholestyramine showed a TC decrease of −34.5%; (III) the group receiving the above drugs together with nicotinic acid showed a decrease of −41.9%; (IV) finally, the group receiving lovastatin, cholestyramine and nicotinic acid showed the greatest decrease in TC levels, i.e., −47.1%. Although patients taking DMSO reported that they had an unpleasant mouth odour, which in most cases remitted with chlorophyll, most adverse effects were associated with nicotinic acid: 20% of patients (80% in the DMSO + lovastatin + cholestyramine + nicotinic group and 20% in the lovastatin + cholestyramine + nicotinic acid arm) showed redness and 16% (60% in the DMSO + lovastatin + cholestyramine + nicotinic group and 20% in that receiving lovastatin + cholestyramine + nicotinic acid) developed acanthosis nigricans. Other side effects such as hepatic enzyme changes, night-time agitation or constipation (due to cholestyramine) were also reported.
Discussion
The clinical trials assessing potential treatments for NP disease have focused on investigating the effects of miglustat, hydroxypropyl-β-cyclodextrin (HPB-CD) or other drugs or drug combinations in neurological disease progression or blood cholesterol profile, and only in NPC type. Unfortunately there is no uniformity among the different study outcomes, making it very difficult to conclude which is the most appropriate therapy that is currently available for NP. In addition, we are not aware of any published clinical trial that has studied potential treatments for NPA or NPB.
Miglustat was originally approved for the treatment of GD and it was not until January 2009 that the European Union approved its use for the treatment of NPC (7). Miglustat inhibits glucosylceramide synthase, the enzyme responsible for catalysing the first step in the synthesis of most glycosphingolipids that accumulate in NPC (19), thereby decreasing lysosomal storage (24). This drug is able to cross the blood-brain barrier and delay the neurological manifestations in both adult and paediatric NPC patients (13,24). In the scientific literature, clinical trials on NP are scarce, and some observational studies have reported on the effects of miglustat in NPC patients (20,31,32).
Preliminary findings by Galanaud and co-workers reported that miglustat had some beneficial effect on brain dysfunction in NPC after a 24-month treatment (33). Findings of clinical trials are in agreement in that miglustat can slow the progression of neurological symptoms in all patients, yet the therapeutic benefit is greater in those with a late diagnosis (i.e., late childhood onset or juvenile/adult onset) compared with early childhood onset. On the other hand, there is no uniformity among published trials in the presentation of results. Most (n=4) but not all of the 6 selected studies showed the time course of the disease (i.e., % of improvement stagnation or deterioration) in different neurological parameters (HSEM, cognition, ambulation, swallowing). In addition, there are differences among studies in the neurological parameters reported. Furthermore, results within a study are not always shown for all trial groups, hampering potential comparisons between them. Several adverse effects associated with miglustat such as diarrhea, flatulence, intestinal carbohydrate malabsorption and weight loss must be also underlined (12,13,17,24,26,29,34,35).
Other forms of treatment are based on the use of cholesterol-lowering drugs or low cholesterol diets for the reduction of hepatic cholesterol. The trial performed by Patterson and co-workers (28) was prior to the approval of miglustat as a therapy for NPC [2009]. Thus, the authors studied the effects of diet, DMSO (for its effects on cholesterol transport) and 3 drugs frequently used for the treatment of hypercholesterolemia, i.e., lovastatin, cholestyramine and nicotinic acid (28). The study showed important decreases in plasma and liver cholesterol levels, mainly due to the combination of lovastatin, cholestyramine and nicotinic acid. Yet no information was reported on neurological progression.
Unfortunately no treatment has yet proven able to change the actual course of NPC. Meanwhile, miglustat is the first and only specific drug approved for this disease in Europe [2009], Canada [2010] and Japan [2012]; its objective is based on alleviating disease symptoms while attenuating neurodegeneration (13,19). In the US, the FDA declined to approve this drug in 2010 and called for more data. The good news is that enzyme replacement therapy might represent a more promising treatment and there are currently two ongoing trials with recombinant human ASM for adults and elderly with NPB (EudraCT reference numbers 2010-023953-12 and 2013-000051-40). There is also an ongoing trial with N-butyldeoxynojirimycin (NB-DNJ)-miglustat in NPC patients of all ages (EUdraCT number 2006-005842-35), and another one (phase 1/2 study still in recruitment phase -NCT02124083) for NPC patients aged 18 to 60 years with vorinostat (a histone deacetylase inhibitor that has been shown in vivo to increase mutant NPC1 protein levels and to reverse cellular accumulation of unesterified cholesterol).
In summary, at present there are only published clinical trials investigating the treatment for one specific type of NP disease, NPC. Furthermore, there is no uniformity among study outcomes nor in the way the results were analysed and presented by the different authors. Miglustat is expected to delay the neurological symptoms of NPC, with greater benefits in patients with a late onset of the disease. HPB-CD is also able to attenuate clinical symptoms although it is not possible to compare the effectiveness of the two compounds owing to the limited data available for the latter. As for cholesterol-lowering drugs, the combination of lovastatin, cholestyramine and nicotinic acid is the most effective one for lowering cholesterolemia. Owing to the low number of clinical trials assessing NP treatment and the lack of additional information, it is not yet possible to make a comparison between the different types of treatments for this disease.
Acknowledgements
Funding: G Nogales-Gadea is supported by a Miguel Servet grant ISCIII CD14/00032, PI15/01756 and FEDER. Research studies by Alejandro Lucia are funded by the Fondo de Investigaciones Sanitarias (FIS, grant number PI12/00914) and cofinanced by Fondos FEDER.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stern G. Niemann-Pick’s and Gaucher’s diseases. Parkinsonism Relat Disord. 2014;20 Suppl 1:S143-6. [PubMed]
- Pérez-Sala D, Boya Tremoleda P, Stamatakis K. Use of a protein sequence of localisation and endolysosomal degradation. Available online: http://digital.csic.es/bitstream/10261/27861/1/WO2010034870A1.pdf
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000.
- Guadalupe JT, Fabiola OP, David IG. Enfermedad de Niemann-Pick tipo C. Revista Mexicana de Neurociencia 2012;13:281-5.
- Wang RY, Bodamer OA, Watson MS, et al. Lysosomal storage diseases: diagnostic confirmation and management of presymptomatic individuals. Genet Med 2011;13:457-84. [PubMed]
- Crocker AC. The cerebral defect in Tay-Sachs disease and Niemann-Pick disease. J Neurochem 1961;7:69-80. [PubMed]
- Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis 2010;5:16. [PubMed]
- Brady RO, Kanfer JN, Mock MB, et al. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proc Natl Acad Sci U S A 1966;55:366-9. [PubMed]
- Thurberg BL, Wasserstein MP, Schiano T, et al. Liver and skin histopathology in adults with acid sphingomyelinase deficiency (Niemann-Pick disease type B). Am J Surg Pathol 2012;36:1234-46. [PubMed]
- McGovern MM, Wasserstein MP, Giugliani R, et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics 2008;122:e341-9. [PubMed]
- Marfa MP. Enfermedad de Niemann-Pick C. Barcelona: Publicaciones Permanyer, 2012.
- Pineda M, Perez-Poyato MS, O'Callaghan M, et al. Clinical experience with miglustat therapy in pediatric patients with Niemann-Pick disease type C: a case series. Mol Genet Metab 2010;99:358-66. [PubMed]
- Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab 2010;99:351-7. [PubMed]
- Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early-stage lung adenocarcinomas are associated with unique clinicopathologic and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287-94. [PubMed]
- The GeneCards Human Gene Database. Available online: http://www.genecards.org/cgi-bin/carddisp.pl?gene=NPC2&search=NPC2
- Alavi A, Nafissi S, Shamshiri H, et al. Identification of mutation in NPC2 by exome sequencing results in diagnosis of Niemann-Pick disease type C. Mol Genet Metab 2013;110:139-44. [PubMed]
- Héron B, Valayannopoulos V, Baruteau J, et al. Miglustat therapy in the French cohort of paediatric patients with Niemann-Pick disease type C. Orphanet J Rare Dis 2012;7:36. [PubMed]
- Wraith JE, Guffon N, Rohrbach M, et al. Natural history of Niemann-Pick disease type C in a multicentre observational retrospective cohort study. Mol Genet Metab 2009;98:250-4. [PubMed]
- Wraith JE, Imrie J. New therapies in the management of Niemann-Pick type C disease: clinical utility of miglustat. Ther Clin Risk Manag 2009;5:877-87. [PubMed]
- Pineda M, Wraith JE, Mengel E, et al. Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol Genet Metab 2009;98:243-9. [PubMed]
- Matsuo M, Togawa M, Hirabaru K, et al. Effects of cyclodextrin in two patients with Niemann-Pick Type C disease. Mol Genet Metab 2013;108:76-81. [PubMed]
- Abel LA, Bowman EA, Velakoulis D, et al. Saccadic eye movement characteristics in adult Niemann-Pick Type C disease: relationships with disease severity and brain structural measures. PLoS One 2012;7:e50947. [PubMed]
- Lyseng-Williamson KA. Miglustat: a review of its use in Niemann-Pick disease type C. Drugs 2014;74:61-74. [PubMed]
- Patterson MC, Vecchio D, Prady H, et al. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol 2007;6:765-72. [PubMed]
- Masciullo M, Santoro M, Modoni A, et al. Substrate reduction therapy with miglustat in chronic GM2 gangliosidosis type Sandhoff: results of a 3-year follow-up. J Inherit Metab Dis 2010;33 Suppl 3:S355-61. [PubMed]
- Belmatoug N, Burlina A, Giraldo P, et al. Gastrointestinal disturbances and their management in miglustat-treated patients. J Inherit Metab Dis 2011;34:991-1001. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [PubMed]
- Patterson MC, Di Bisceglie AM, Higgins JJ, et al. The effect of cholesterol-lowering agents on hepatic and plasma cholesterol in Niemann-Pick disease type C. Neurology 1993;43:61-4. [PubMed]
- Patterson MC, Vecchio D, Jacklin E, et al. Long-term miglustat therapy in children with Niemann-Pick disease type C. J Child Neurol 2010;25:300-5. [PubMed]
- Fecarotta S, Romano A, Della Casa R, et al. Long term follow-up to evaluate the efficacy of miglustat treatment in Italian patients with Niemann-Pick disease type C. Orphanet J Rare Dis 2015;10:22. [PubMed]
- Chien YH, Peng SF, Yang CC, et al. Long-term efficacy of miglustat in paediatric patients with Niemann-Pick disease type C. J Inherit Metab Dis 2013;36:129-37. [PubMed]
- Ginocchio VM, D'Amico A, Bertini E, et al. Efficacy of miglustat in Niemann-Pick C disease: a single centre experience. Mol Genet Metab 2013;110:329-35. [PubMed]
- Galanaud D, Tourbah A, Lehéricy S, et al. 24 month-treatment with miglustat of three patients with Niemann-Pick disease type C: follow up using brain spectroscopy. Mol Genet Metab 2009;96:55-8. [PubMed]
- Amiri M, Naim HY. Miglustat-induced intestinal carbohydrate malabsorption is due to the inhibition of α-glucosidases, but not β-galactosidases. J Inherit Metab Dis 2012;35:949-54. [PubMed]
- Santos ML, Raskin S, Telles DS, et al. Treatment of a child diagnosed with Niemann-Pick disease type C with miglustat: a case report in Brazil. J Inherit Metab Dis 2008;31 Suppl 2:S357-61. [PubMed]