Charged particles for liver cancer
Hepatocellular carcinoma (HCC), representing approximately 90% of all liver cancers, has a dismal prognosis: only 10–20% of the tumors can be completely removed by surgery. Cancer progression is the main cause of death, while distal metastasis are seldom even in locally advanced, unresectable HCC. Therefore, loco-regional therapies are essential. Several therapeutic options are available for HCC in addition to surgery, such as embolization or thermal ablation. For large tumors, unfortunately recurrence remains high (1).
Radiotherapy has limited applications, due to the low radiation tolerance of the liver, while high doses are needed for tumor sterilization. The risk of developing radiation-induced liver disease (RILD) is about 5–10% when the whole liver is irradiated up to 30–35 Gy, but the radiation dose to control most HCC is around 50–70 Gy. Recent technologies in radiotherapy can be used to maximize the effect on the tumor with acceptable toxicity. The National Comprehensive Cancer Network (NCCN) guidelines indicate that radiotherapy, especially stereotactic body radiotherapy (SBRT), may represent an alternative to percutaneous or transarterial interventions in unresectable HCC (2).
Charged particles offer a favourable depth-dose distribution compared to X-rays, and are therefore ideal for sparing normal tissue in radiotherapy (3). Charged particle therapy (CPT) is generally acknowledged as the best therapeutic option for a few selected solid tumors, such as uveal melanoma, chordomas and chondrosarcomas of the base of the skull, and pediatric tumors. Several trials are currently ongoing on the use of energetic ions in tumors with high prevalence, including gastrointestinal cancers. The recent Proton Beam Therapy Model Policy of the American Society for Radiation Oncology (ASTRO) includes HCC in the Group 1 (medically necessary), when used in hypofractionation (4). Accelerated carbon ions are also be used in HCC in Asia and Europe. The Japanese results for large tumours near the porta hepatis reflect a major progress in treatment of HCC because no efficient alternatives are available (5).
Qi and co-workers (6) have now published a very comprehensive review and meta-analysis of CPT for HCC in comparison with X-ray therapy, including SBRT. Compared to previous reviews (7-9), this work extracted quantitative information for a direct comparison of three radiotherapy options. No randomized controlled trials are available for a direct comparison of CPT and X-rays, but the authors identified 70 non-comparative, observational studies, describing 3,577 patients in 53 cohorts treated with X-rays and 20 cohorts (1,627 patients) with CPT. The authors analyzed overall survival, progression-free survival, local control, acute and late toxicity. They divided the treatments in three main groups: CPT, SBRT and conventional radiotherapy (CRT), the latter including 3D conformal radiotherapy (3DCRT), image-guided radiotherapy (IGRT), and intensity modulated radiotherapy (IMRT). The results for tumor control and toxicity are summarized in Figure 1 and Figure 2, respectively. The main conclusion is that survival rates are significantly higher for CPT than for CRT, but similar to SBRT. However, CPT has lower toxicity than X-ray treatments.
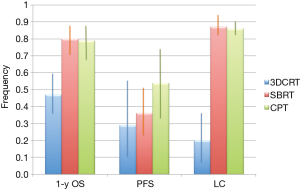
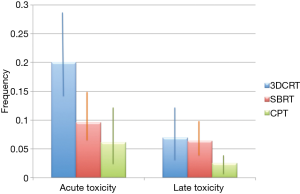
The meta-analysis supports to the hypothesis that CPT is the most effective radiotherapy option for HCC. However, no firm conclusions can be extracted from the current data: radical and palliative treatments were pooled, and the data on CPT are still limited (10). A comparison between observation studies may have biases that can only be resolved with phase-III comparative trials of CPT vs. SBRT and/or CRT. One problem in such a trial is the number of arms: radiation quality (protons, C-ions, X-rays), total dose, and fractionation. For instance, SBRT vs. CRT would compare different fractionation with similar dose distribution and radiation quality, but SBRT vs. C-ions changes both fractionation and physical dose distributions. A comparison of the ranges of total doses and number of fractions used in CPT, SBRT and CRT is shown in Figure 3. Clearly SBRT and C-ion therapy are exploring extreme hypofractionation, while protontherapy and CRT use more conventional fractionation schemes. There is a potential for hypofractionation in protontherapy, only marginally explored so far in a few studies reaching 4-8 fractions with total doses around 50 Gy.
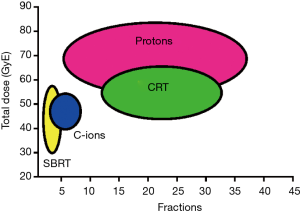
At the moment, there are no active phase-III trials protons vs. X-rays for HCC, and only a handful for other sites. Phase-II trials on proton therapy hypofractionation in HCC are ongoing at the National Cancer Center (NCC) in Korea and Massachusetts General Hospital (MGH) in USA. Randomized controlled trials of transarterial chemoembolization or radiofrequency ablation vs. proton beam radiotherapy are recruiting patients at Loma Linda University, USA, and NCC, Korea, respectively. These trials are very important to determine the feasibility of hypofractionation in HCC CPT, and to determine the role of protons, and radiotherapy in general, in the treatment of HCC. The next step must be a comparative trial of protons vs. SBRT or CRT using the same fractionation schemes.
For C-ions, phase-III trials are even more rare, and being heavy ions even much more expensive than protons this lack of level-I evidence is considered the main hindrance to a more widespread use of C-ions in oncological practice. The US National Cancer Institute has recently proposed an International phase-III trial C-ions vs. IMRT for locally advanced pancreatic cancer, based on the excellent results obtained in Japan (14). Pancreatic cancer is easy to metastasize and therefore local treatments are always combined to chemotherapy. Moreover, treatment of the pancreas with pencil beam scanning is complicated by the complex organ movement, and ideally the treatment plan requires the use of a gantry to find optimal angles. HCC may be an excellent alternative for a phase-III trial. Both in Japan (11) and Germany (PROMETHEUS-01 trial) (12), HCC is treated by C-ions in only 4 fractions up to 40-52.8 GyE. This is consistent with the schedule used in SBRT, and therefore a trial having as primary endpoints survival, local control, and toxicity should be able to clarify whether heavy ions have an advantage.
In conclusion, the meta-analysis of Qi et al. (6) suggests that CPT can play an important role in the treatment of HCC. Prospective randomized clinical trials comparing protons or C-ions to SBRT or IMRT should be performed to gain evidence of the superiority of particles compared to X-rays. A scheme of 40-52 Gy in 4 fractions is frequent in SBRT (8) and C-ion therapy (11,12), and it has been also used in a few patients with protons at Hyogo (13). Protons and C-ions have similar physical dose distribution, but different biological properties in the target volume. The results from Hyogo show similar local control at the same total dose (13). SBRT and protons have similar biological effects, but different physical dose distribution. Particle beam scanning can be used effectively with this treatment regime, because the interplay effect caused by motion is mitigated by the fractionation in four split doses (15). Therefore, using the same dose and fractionation scheme, a 3-arms trial should clarify the importance of the reduced dose outside the target (in the normal tissue) and different radiation quality in the target. Liver may be one of the best sites to conduct conclusive comparative clinical trials on the advantages of charged particles in oncology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 2012;62:394-9. [PubMed]
- NCCNN, Clinical Practice Guidelines in Oncology for Hepatobiliary Cancers. Version 2.2012. Available online: www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Loeffler JS, Durante M. Charged particle therapy--optimization, challenges and future directions. Nat Rev Clin Oncol 2013;10:411-24. [PubMed]
- ASTRO, Model Policies: Proton Beam Therapy. American Medical Association, 2014. Available online: https://www.astro.org/uploadedFiles/Main_Site/Practice_Management/Reimbursement/ASTRO%20PBT%20Model%20Policy%20FINAL.pdf
- Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015;16:e93-e100. [PubMed]
- Qi WX, Fu S, Zhang Q, et al. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 2015;114:289-95. [PubMed]
- Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013;87:22-32. [PubMed]
- Sanuki N, Takeda A, Kunieda E. Role of stereotactic body radiation therapy for hepatocellular carcinoma. World J Gastroenterol 2014;20:3100-11. [PubMed]
- Dionisi F, Widesott L, Lorentini S, et al. Is there a role for proton therapy in the treatment of hepatocellular carcinoma? A systematic review. Radiother Oncol 2014;111:1-10. [PubMed]
- Yamazaki H, Nakamura S, Suzuki G, et al. Superiority of charged particle therapy in treatment of hepatocellular carcinoma (Regarding Qi W.X. et al. charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis). Radiother Oncol 2015. [Epub ahead of print]. [PubMed]
- Imada H, Kato H, Yasuda S, et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol 2010;96:231-5. [PubMed]
- Habermehl D, Debus J, Ganten T, et al. Hypofractionated carbon ion therapy delivered with scanned ion beams for patients with hepatocellular carcinoma - feasibility and clinical response. Radiat Oncol 2013;8:59. [PubMed]
- Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011;117:4890-904. [PubMed]
- Durante M, Tommasino F, Yamada S. Modeling Combined Chemotherapy and Particle Therapy for Locally Advanced Pancreatic Cancer. Front Oncol 2015;5:145. [PubMed]
- Richter D, Saito N, Chaudhri N, et al. Four-dimensional patient dose reconstruction for scanned ion beam therapy of moving liver tumors. Int J Radiat Oncol Biol Phys 2014;89:175-81. [PubMed]


