Relationship between serum CA19-9 and CEA levels and prognosis of pancreatic cancer
Introduction
Pancreatic cancer (PC) is a highly malignant tumor in the digestive system, with its incidence progressively increasing in recent years (1,2). Due to the lack of specific clinical symptoms in its early stages, PC is often confirmed in its advanced stage. The prognosis is poor: PC patients have a mean survival of 6-8 months and a 5-year survival rate of less than 5% (3). The committee of PC under the Chinese Society of Clinical Oncology (CSCO) retrospectively analyzed 2,340 PC cases and found the 1-, 3-, and 5-year survival rates among PC patients who had undergone radical resection were 54.4%, 13.5%, and 8.5%, respectively (4). Early diagnosis and appropriate therapies based on the prognosis are essential to increase the survival rate among PC patients. Some tumor-related antigens can be used to diagnose PC and reflect its possible progression. CA19-9 and CEA are two commonly used markers for diagnosing PC. In our current study, we investigated the possible effects of serum CA19-9 and CEA expressions on the prognosis of PC patients.
Materials and methods
General data
The clinicopathological data of 128 patients with pancreatic adenocarcinoma who were treated in our center between January 2012 and December 2013 were retrospectively analyzed. These patients included 79 men and 49 women aged 42-82 years (mean, 62 years). Primary pancreatic ductal adenocarcinoma was confirmed by postoperative pathology in all cases. The tumor was located at the head of the pancreas in 99 patients and at the tail of the pancreas in 29 patients. The degree of tumor differentiation, lymph node metastasis, and tumor size were determined by postoperative pathology. Highly differentiated adenocarcinoma accounted for 40.7% (n=50), and moderately and poorly differentiated adenocarcinoma 59.3% (n=73). Tumor size was based the maximal diameters, which were tasis, and tumor size were determined by postoperative pathology. Highly differentiated adenocarcinoma accounted fwas seen in 66 cases (51.6%). The clinical staging was based on the AJCC Cancer Staging Manual (7th Edition) [2010] for PC (5). The number of patients in stages I, IIA, IIB, III, and IV was 7, 52, 61, 6, and 2, respectively.
Methods
Peripheral fasting venous blood samples were collected before surgery, and serum was obtained by routine centrifuging. Serum CA19-9 and CEA levels were determined by electrochemiluminescence immunoassay (double-antibody sandwich ELISA) on a Roche cobas e 602 module. Roche’s original ancillary reagents were applied, which included streptavidin-coated magnetic particles, biotinylated anti-CA19-9 and anti-CEA monoclonal antibodies, and Ru-labelled anti-CA19-9 and anti-CEA monoclonal antibodies. The cut-off values for serum CA19-9 and CEA levels were 39 U/mL and 4.7 ng/mL, respectively.
Patient inclusion criteria: (I) patients who had undergone complete surgical resection of the tumor (e.g., pancreas-duodenum resection and resection of pancreatic body and tail); and (II) pancreatic ductal adenocarcinoma was confirmed by postoperative pathology. Exclusion criteria: (I) with a history of other malignancy before surgery; (II) preoperative adjuvant chemotherapy; and (III) had received a palliative operation, and the tumor was not removed (e.g., bile duct-jejunum anastomosis). Follow-up was conducted by telephone, mails, and household visits. The deadline of the follow-up was December 30, 2014, with the follow-up endpoint being the death of patient.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software. The clinical data including age, gender, pathological conditions, and serum CA19-9 and CEA levels were retrospectively analyzed. Univariate analysis was based on the Kaplan-Meier curves, and comparison of survivals among different groups was based on Log-rank test. During the multivariate analysis, Cox proportional hazard model was applied for regression analysis. Stepwise selection was applied for model variables, with the entry probability being <0.05 and the removal probability being >0.10. A value of P <0.05 was considered statistically significant.
Results
Survivals
Among these 128 PC patients, the median survival was 12.2 months, and the 6-month and 1-year survival rates were 87.5% and 56.8%, respectively.
Effects of CA19-9 and CEA on the prognosis of PC patients
Of these 128 patients, serum CA19-9 and CEA expression levels were increased in 100 patients (78.1%) and 48 patients (37.5%), respectively. The median survival was 16.5 months (range, 4.2-33.2 months) in patients with normal CA19-9 level and was 12.2 months (range, 1.7-32.2 months) in patients with elevated CA19-9 level; survival curves showed significantly better prognosis in normal group than in elevated group (P=0.027, Figure 1A). Compared with patients with normal CEA level, patients with elevated CEA level had significantly shorter survival (10.3 vs. 14.4 months; P=0.036) (Figure 1B).
We further divided all these 128 PC patients into three groups according to the serum CA19-9 and CEA levels: group A: both CA19-9 and CEA were normal; group B: either CA19-9 or CEA was elevated; and group C: both CA19-9 and CEA were elevated. The median survival in groups A, B, and C was 17.5, 12.2, and 10.3 months. The prognosis was significantly superior in group A than in groups B and C (group A vs. group B, P=0.007; group A vs. group C, P=0.004) (Figure 1C).
Multivariate Cox regression analysis was then performed with age, gender, tumor site, tumor size, differentiation degree, depth of invasion, lymph node metastasis, distant metastasis, clinical stage, and serum CA19-9 and CEA levels as the independent variables and survival as the dependent variable. It was found that lymphatic metastasis, CA19-9 >39 U/mL, and CEA >4.7 ng/mL were independent prognostic factors in PC patients (Table 1).
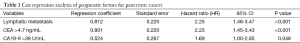
Full table
Discussion
PC is a highly malignant tumor that is difficult to diagnose in its early stages. With no effective treatment, its prognosis is extremely poor. While radical resection remains the main treatment for this malignancy, most patients were already in advanced stages when the disease is confirmed and thus lose the opportunity for surgical treatment. Further, surgical resection alone is accompanied by high incidence of recurrence. Clinically, monitoring of some circulating tumor markers is helpful to identify tumor occurrence and progression. Among them CA19-9 and CEA are two commonly used markers for PC (6,7).
CA19-9 is a glycolipid and belongs to the sialyl-Lewisx blood group antigens. In PC patients, it is present in serum in the form of mucin (8). CA19-9 has high sensitivity (61-86%) in the diagnosis of PC. Currently it is the most sensitive marker for PC (9). In addition, it has been reported that preoperative serum CA19-9 expression level in PC patients might be correlated with tumor resectability and postoperative tumor progression (10,11). CEA is an acidic glycoprotein characteristic of fetal antigens. It is often used for the diagnosis of colorectal cancer. It has relatively low sensitivity and specificity for PC diagnosis; in clinical settings, it is often combined with other tumor markers (12).
In our current study, we found that the abnormal serum CA19-9 and CEA levels were closely correlated with the prognosis of PC patients. In addition, compared with patients with normal CA19-9 and CEA levels, patients with elevated CA19-9 and/or CEA levels often had poorer prognosis. This may because the elevated CA19-9 and CEA levels indicate that the tumor has already in its advanced stage; furthermore, the levels of tumor markers increase along with tumor progression. It has been reported (13,14) that the CA19-9 and CEA levels were closely correlated with the differentiation degree, lymph node metastasis, and tumor progression in PC patients. Notably, serum CA19-9 level can also increase in patients with some benign diseases (e.g., biliary obstruction, pancreatitis, and liver cirrhosis). Cox logistic analysis in our study further revealed that lymphatic metastasis, CA19-9 >39 U/mL, and CEA >4.7 ng/mL were independent prognostic factors in PC patients. Although the positive rate of CEA was lower in PC patients in our study, its increase often means poorer prognosis.
Quite a few articles in China (4,15,16) have also reported the prognostic factors of PC; however, most patients in these studies were already in later stages and patients had received adjuvant chemotherapy and/or palliative surgery. In contrast, all the patients in our current study received radical resection of the tumor. Due to its unique biologic and anatomic features, PC can easily invade its adjacent nerves, portal vein, and superior mesenteric artery and vein. Thus, surgical operations can be extremely challenging and is often contraindicated. Therefore, most patients enrolled in our study were in stage II. For these patients who were still operateable, the prognosis could be predicted based on preoperative serum CEA and CA19-9 levels. Nevertheless, in this retrospective study, the clinical data of some PC patients were missing, and biases and errors might exist. Multicenter prospective cohort studies with larger sample sizes are warranted to validate our findings.
In summary, many factors may affect the prognosis of PC patients. The biological features of PC determine the postoperative survival. Preoperative serum CA19-9 and CEA levels may be auxiliary indicators for predicting the prognosis of PC patients. Patients with high CA19-9 and CEA levels should be carefully and reasonably managed.
Acknowledgements
Funding: This study was supported by National Natural Science Foundation of China (81371894, 81302531, 81272324, 81201359, 81101322), Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin 2012;62:283-98. [PubMed]
- Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir 2004;59:99-111. [PubMed]
- Zhang QH, Ni QX. Coordination Group of The Committee on Pancreatic Cancer. Clinical analysis of 2340 cases of pancreatic cancer. Zhonghua Yi Xue Za Zhi 2004;84:214-8. [PubMed]
- Pancreatic Surgery Group of Surgery Branch of Chinese Medical Association. Guideline for the diagnosis and treatment of pancreatic adenocarcinoma (2014 edition). Chinese Journal of Digestive Surgery 2014;13:831-7.
- Ni XG, Bai XF, Mao YL, et al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol 2005;31:164-9. [PubMed]
- Chang MC, Liang PC, Jan S, et al. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels. Pancreatology 2014;14:366-72. [PubMed]
- Rosty C, Goggins M. Early detection of pancreatic carcinoma. Hematol Oncol Clin North Am 2002;16:37-52. [PubMed]
- Huang Z, Liu F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a meta-analysis. Tumour Biol 2014;35:7459-65. [PubMed]
- Halloran CM, Ghaneh P, Connor S, et al. Carbohydrate antigen 19.9 accurately selects patients for laparoscopic assessment to determine resectability of pancreatic malignancy. Br J Surg 2008;95:453-9. [PubMed]
- Liu L, Xu H, Wang W, et al. A preoperative serum signature of CEA+/CA125+/CA19-9 ≥ 1000 U/mL indicates poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer 2015;136:2216-27. [PubMed]
- Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA199, CA125, CEA, and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell Biochem Biophys 2014. [Epub ahead of print]. [PubMed]
- Lucarotti ME, Habib NA, Kelly SB, et al. Clinical evaluation of combined use of CEA, CA19-9 and CA50 in the serum of patients with pancreatic carcinoma. Eur J Surg Oncol 1991;17:51-3. [PubMed]
- Lee KJ, Yi SW, Chung MJ, et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J 2013;54:643-9. [PubMed]
- Wang H, Zhi F, Zhao X, et al. Diagnosis and prognosis of 280 patients with pancreatic carcinoma. Chinese Journal of Pancreatology 2010;10:2-5.
- Fu C, Huang H, Chen Y, et al. Multiplicity of prognosis of pancreatic carcinoma patients. International Journal of Surgery 2013;40:303-6.



