Pre-operatively misdiagnosed undifferentiated embryonal sarcoma of the liver: analysis of 16 cases
Introduction
Undifferentiated embryonal sarcoma of the liver (UESL), also known as malignant mesenchymal tumor, embryonal sarcoma (1), or fibromyxoid sarcoma, is an extremely rare malignancy that arises from liver mesenchymal tissue. It is highly malignant and invasive, ranking the fourth among childhood liver tumors (after hepatoblastoma, infantile hemangioendothelioma, and liver cancer) (2). It accounts for about 13% of pediatric primary liver cancers (3), particularly in populations aged 6-10 years (90% of the patients are younger than 15 years) (4). Since UESL is mainly seen in young children and adolescents (5) and is rare among adults, its clinical symptoms in the early stage may not by typical, and its laboratory and radiological findings are also non-specific. The prevalence of hepatitis is high in China. In the adult UESL patients, the disease is often accompanied by hepatitis, cirrhosis, or liver dysfunction, making the pre-operative diagnosis of UESL extremely difficult. As a result, UESL is often misdiagnosed as other tumors, and the patients often have a short survival after the disease is finally confirmed (6). In our current study, we analyzed the clinical and imaging data of 16 UESL patients whose disease was misdiagnosed during the period from 2009 to 2014, with an attempt to improve the pre-operative diagnosis accuracy.
Subjects and methods
Data collection
The data of 47 patients with pathologically confirmed UESL from 2009 to 2014 were retrospectively analyzed. Among them 26 had incomplete imaging data, 3 sought treatment in our hospital after disease relapse, and 2 were correctly diagnosed before surgery. The disease was misdiagnosed before surgery in 16 patients, among whom 9 were misdiagnosed as primary liver cancer, 3 as hepatoblastoma, and 4 as malignant hepatic mass.
Examinations
GE 730, AU 4, or SEQUIA ultrasound diagnostic machines were used for routine two-dimensional examinations, with the probe frequency set at 2-5 MHz.
LightSpeed QX/I multi-slice spiral CT machine was used for CT scanning. A plain scan of the whole liver was performed for 16-20 s, followed by triple-phase contrast-enhanced scans. The contrast agent used was iohexol (1.5 mL/kg), which was injected via the elbow vein at a speed of 2.5-3.5 mL/s, followed by the scanning of the arterial phase (23-26 s), portal phase (50-60 s) and delayed phase (120-140 s).
MRI was performed using Signa Inifinity Twin Speed 1.5T MRI machine (GE, USA). The T1-weighted imaging (T1WI) used the fast spoiled gradient-echo (FSPGR) sequence (duration: 16-20 s), T2-weighted imaging (T2WI) cross-sectional scans were performed using fast spin-echo (FSE) in addition to fat suppression sequence (120-180 s). Diffusion-weighted imaging (DWI) was performed using spin echo-echo planar imaging (SE-EPI) sequence. The contrast agent used in the triple-phase dynamic MR enhanced scans was Gd-DTPA (dose: 0.1 mmoL/kg; delivered at 2.0-3.0 mL/s); after the contrast agent was injected via the elbow vein, the arterial phase (20-25 s), portal venous phase (55-65 s) and delayed phase (120-160 s) were scanned.
Pathological examination
The specimen was fixed in 10% neutral-buffered before routine dehydration and dewaxing; serial paraffin-embedded tissue slices were made for routine HE staining and for immunohistochemical staining. An EnVision (Dako) two-step visualization method was applied. The selected antibodies included alpha-fetoprotein (AFP), HepParl, CK18, CK19, Vimentin (VI), HBsAg, CD34, α1-antitrypsin (α1-AT), S-100, CD68, and pCEA.
Image analysis
The imaging data were analyzed by two senior radiologists. The amount, location, shape, size, margins, hemorrhage, necrosis, and degree and mode of enhancement were observed.
Results
Clinical data
The clinical data and laboratory findings of 16 UESL patients are shown in Table 1. Among these patients, there were 12 males and 4 females. Three patients were below 18 years (range, 9-14 years; mean, 11 years), 13 were above 18 years (range, 25-70 years; mean, 51 years). The clinical manifestations at presentation included abdominal pain/abdominal discomfort (n=12), fatigue/loss of appetite (n=1), and chills/fever (n=1). Two patients had no obvious discomfort, and the disease was identified during health check-ups.
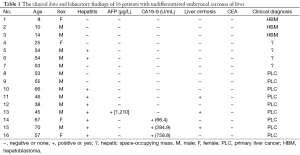
Full table
Pathological findings
Most post-operative tumor specimens were irregular huge masses that were gray-white in color and had sharp edges. Some tumors had capsule, and the capsule was complete in 2 cases and incomplete in 8 cases. The tumors had no obvious capsule in 6 cases. The tumors sized 5.2-21.4 cm (mean, 13.3 cm). The cutting edge was cystic, in which multiple cystic separations could be seen. The cyst contained brown gel-like substances. Varying degrees of hemorrhage and necrosis were seen inside the tumor stroma.
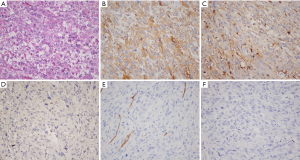
Microscopy showed embryonic mesenchymal differentiation in tumor cells; the sarcoma cells were pleomorphic, showing tight or loose arrangement. The borders among cells were unclear. The nucleus was large and deeply stained, showing obviously irregular shapes. Pathological mitoses and abnormal nuclei were visible, and eosinophil corpuscles were found in the cytoplasm and stroma (Figure 1A). Immunohistochemical findings included: α1-AT (+−+++) (n=16), VI (+−+++) (n=14), CD68 (+−+++) (n=12); AFP (−); Hep Par1 (−); HBsAg (−); pCEA (−); CKl9 (−); CKl8 (−); S-100 (−); and CD34 (−) (Figure 1B-F).
Imaging findings
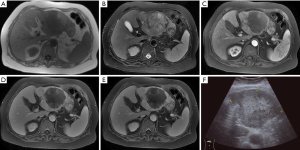
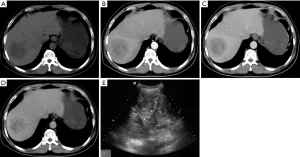
In 9 patients whose disease was misdiagnosed as primary liver cancer, ultrasound showed solid mass-like high-echo shadows in the liver, with non-homogeneous internal echo. Four patients underwent CT, among whom 3 had multiple lesions and 1 had a single lesion. Plain scan showed hypo-echoic mass for each lesion, and liquid area after hemorrhage and necrosis could be seen inside the lesion. The liquid had a density slightly higher than water. Mild heterogeneous enhancement was seen in the tumor after contrast administration. In three cases, Halo sign was seen at the edge of the lesion in the delayed phase. In one patient, the branch of portal vein was invaded, and metastases to the right adrenal gland and right upper lung were also found; in one patient, extensive tumor thrombosis was seen in the portal vein; and in the remaining one patient, metastases to the right kidney and diaphragm were detected. Five patients underwent MRI, among whom 4 patients had a single lesion and 1 patient had a major lesion accompanied by multiple minor lesions inside the liver. On T1WI the lesions showed mixed low signals accompanied by patchy high-signal intensities; on T2WI, there were mixed high-signal intensities. Mild continuous enhancements were seen at the edge and stroma of the lesion after contrast administration, and irregular patchy non-enhanced regions were visible inside the lesions (Figure 2). In 2 cases, the enhancement of the lesion was not obvious in the arterial phase after contrast administration, whereas mild heterogeneous enhancement was seen in the delayed phase. In 2 cases, Halo sign was visible at the edge of lesion in the delayed phase; among them the branches of the portal vein were involved in one patient and tumor thrombosis in the right branch of portal vein was seen in the other patient.
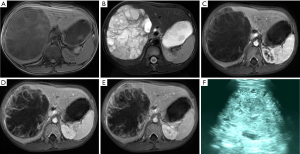
The disease was misdiagnosed as hepatoblastoma in three cases, in whom a single lesion was found in the right liver lobe. Ultrasound showed solid cluttered hyperechoic mass in the liver, with heterogeneous internal echoes. In 3 cases, MRI showed huge mass sized 12.2-14.0 cm in right liver. T1WI showed heterogeneous low-signal intensity in the lesion, accompanied by hemorrhagic patchy high-signal intensities inside the lesion; the borders were clear. Heterogeneous high-signal intensities were seen on T2WI, along with low-signal areas or zones inside the tumor. Mildly and constantly heterogeneous enhancements were seen in the lesion after contrast administration. Halo sign was visible at the tumor edge in the delayed phase (Figure 3).
In 4 cases, the lesions could not be confirmed and were only diagnosed as intrahepatic malignant space-occupying masses. Among them there were solid hyperechoic masses in two cases, along with heterogeneous internal echoes; in 1 case, cystic hypoechoic mass was seen; and in the remaining 1 case, both cystic and solid echoes were detected, along with heterogeneous internal echoes. Two patients underwent CT, among whom 1 had multiple lesions and 1 had a single lesion. The lesion showed heterogeneous low-signal intensities on plain scan, and mild and constant enhancements were seen after contrast administration (Figure 4). Multiple metastases inside the abdominal cavity were seen in 1 patient. Two patients received MRI. On T1WI, the lesion showed low-signal intensities, along with hemorrhagic patchy high-signal intensities inside the lesions. On T2WI, cluttered high-signal intensities were visible, along with multiple irregular separations. The signals were grouped in the liquid area. Enhancements in the cystic wall and separations were visible at the lesion edge after contrast administration. Constant enhancement was seen in the portal vein phase and delayed phase. Halo sign was seen at the edge of these lesions in the delayed phase.
Discussion
UESL is a rare malignancy arising from the primitive mesenchymal tissue of the liver. Due to the lack of characteristic clinical manifestations, its pre-operative diagnosis is particularly difficult. UESL is highly malignant, and the tumor often has a huge size when identified. Surgical resection remains the treatment of choice for this disease. However, due to the high malignancy, rapid growth, and high recurrence/metastasis rate of UESL, patients with this malignancy often have a low long-term survival rate.
Imaging and immunohistochemical features of UESL
Due to the lack of specific clinical manifestations, the pre-operative diagnosis of UES is mainly based on imaging findings. Ultrasound often shows a solid mass that is often accompanied by cystic degeneration, hemorrhage, and necrosis (7). In some cases, both cystic and solid components can be detected. Plain CT scans often show a huge cystic low-signal intensity with heterogeneous intra-tumor signals and clear borders; calcification is rarely seen inside the lesion (8,9). In our current study, no obvious calcification was seen inside the tumor in all 6 patients who had received CT. After contrast administration, enhanced edge after the enhancement of tumor pseudocapsule can be seen in 40% of UESL (10). During MRI, T2WI showed that the tumor was mainly cystic, showing polycystic and separated morphologies; the tumor stroma was separated, whereas myxoid stroma could be seen in low-signal intensities and separations (6,11). By displaying the internal tumor components, MRI is helpful for the pre-operative assessment of UESL. During the dynamic enhancement, the delayed enhancements gradually increased with time and spread from the peripheral area to the center, along with the mild enhancement of soft tissue or separation intensities inside the cysts. A dense and complete border (fibrous pseudocapsule) can be seen at the edge of a UESL; microscopically, it is composed of bile duct, compressed hepatic cell cords, and blood vessels (12). The ultrasound findings may be inconsistent with those of CT and MRI, which may because the gel-like areas inside UESL constantly absorb water, and, as a result, the tumor is displayed as solid echoes on ultrasound and as liquid intensities or signals on CT and MRI. Such inconsistence is a key diagnostic point in the imaging of UESL (10,13).
Eosinophil corpuscles were found in 10 of 16 patients in study. Vimentin and α1-antitrypsin were detected in most cases, suggesting that the tumor cells are primitive mesenchymal cells. In a few cases, positive expressions of smooth muscle actin (SMA) and cytokeratin (CK) and negative expressions of AFP, HBsAg, CD34, and S-100 were found (14), which are helpful to rule out hepatitis B virus infection and tumors originated from liver cells, epithelium, bile ducts, blood vessel, and nerves.
Reasons of diagnosis
Excessive dependence on clinical and laboratory findings
All the 9 patients whose UESL was misdiagnosed as primary liver cancer were adults, among whom 7 had hepatitis B, 3 had concurrent liver cirrhosis, 3 had increased AFP (the normal value is <25 µg/L) and abnormal liver function, and 1 had increased CA199 (the normal value is <37 U/mL) and abnormal liver function. Men are more prone to liver cancer, especially those with hepatitis. Clinically the liver cancer patients often have liver pain, gastrointestinal symptoms, liver cirrhosis, and metastasis, along with increased tumor markers (AFP and CA199). Liver cancer is a predominantly solid tumor. Most hepatocellular carcinoma tumors are hypervascular, which can be remarkably enhanced in the arterial phase after contrast administration and shown as “fast in and fast out”; also, enhancement of tumor capsule can also be seen in the delayed phase. In contrast, the cholangiocarcinoma is mainly shown as enhanced edge and delayed enhancement in the arterial phase, while hemorrhage and necrosis is rarely seen inside the tumor; in addition, no obvious capsule enhancement is seen in the delayed phase, although Halo sign may be observed. While the imaging finds of these 9 patients did not meet the radiological features of primary liver cancer, a wrong diagnosis of primary liver cancer was made only based on clinical and laboratory findings.
Lack of awareness or experience
All the 4 patients who were misdiagnosed as with intrahepatic space-occupying lesions were adult. Laboratory tests showed no obvious abnormality. Imaging also did not identify the nature of the lesion; rather, it only suggested that there was a space-occupying mass. No definite diagnosis was made before surgery. Three patients who were misdiagnosed as with hepatoblastoma aged 9, 14, and 10 years. These three patients were presented due to abdominal discomfort and intrahepatic huge space-occupying mass. Laboratory tests showed no obvious abnormality. In pediatric patients, UESL often occurs at 6-10 years (i.e., old children and adolescents) (15), with a similar incidence between males and females. In contrast, hepatoblastoma is the most common malignancy in young children, mainly seen in children under 6 years old, with a male: female ratio of 1.5:1 (2). They have similar clinical manifestations including abdominal distension, abdominal pain, and abdominal discomfort, which are mainly due to the compression caused by enlarged tumor body. However, pediatric patients with hepatoblastoma can also have anorexia, emaciation, and anemia; in some male pediatric patients, sexual precocity-related signs and symptoms may also exist. AFP level in hepatoblastoma patients is associated with tumor size and disease course, and the hepatoblastoma patients often have abnormal liver function (2). Radiology of hepatoblastoma may show a predominantly solid tumor with clear border, along with hemorrhage and necrosis inside the tumor. Pseudocapsule is common, and about 50% of hepatoblastoma can have dot-/stripe-like, round, or irregular calcifications. Although the hepatoblastoma has various enhancement modes, it is mainly enhanced during the arterial phase and its intensities are gradually decreased in the portal venous phase and delayed phase. Vascular invasion and tumor thrombus formation are rare in hepatoblastoma.
Measures for reducing misdiagnosis
Measures for reducing misdiagnosis include: (I) detailed history-taking; (II) increased awareness of UESL among clinicians; (III) more knowledge on the radiological features of UESL; and (IV) comprehensive analysis of the results of multiple imaging modes for complex intrahepatic tumors.
Conclusions
In summary, UESL is a rare malignant tumor with the low incidence and clinical diagnosis rate, the high mortality rate and the less recognition. Those descriptions above are expected to understand the disease, help clinicians and improve the diagnostic work in future.
Acknowledgements
The authors would like to thank the other members of the department of radiology for their valuable comments.
Funding: This study was financially supported by the Projects of the Science and Technology Foundation of Shanghai (Grant No. 14DZ1941604).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stocker JT, Ishak KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 1978;42:336-48. [PubMed]
- Yang HF, Zhang XM, Chen XR, et al. Embryonalsarcoma. In: Chen XR, Chen JR, editors. Radiology of Alimentary System. Shanghai: Shanghai Scientific & Technical Publishers, 2010:642.
- Weinberg AG, Finegold MJ. Primary hepatic tumors of childhood. Hum Pathol 1983;14:512-37. [PubMed]
- Lyu S, Shi X, Liang Y, et al. Diagnosis and therapy of primary undifferentiated embryonal sarcoma of the liver. Chin Med J (Engl) 2014;127:1585-7. [PubMed]
- Moon WK, Kim WS, Choi BI, et al. Undifferentiated embryonal sarcoma of the liver treated with chemotherapy: CT imaging in four patients. Abdom Imaging 1995;20:133-7. [PubMed]
- Legou F, Ayav A, Cahn V, et al. Radiologic-pathologic comparison of undifferentiated embryonal sarcoma of the liver in a 61-year-old woman. Diagn Interv Imaging 2012;93:e208-11. [PubMed]
- Xie ZY, Li LP, Wu WJ, et al. Undifferentiated embryonal sarcoma of the liver mistaken for hepatic abscess in an adult. Oncol Lett 2014;8:1184-6. [PubMed]
- Wang XM, Xu SY, He LJ, et al. Undifferentiated embryonal sarcoma of the liver: CT findings. Chin J Radiol 2001;35:380-2.
- Chong ZJ, He SJ, Yin WW, et al. Helical CT findings of undifferentiated embryonal sarcoma of the liver. Chin J HepatobiliarySurg 2007;13:209-10.
- Buetow PC, Buck JL, Pantongrag-Brown L, et al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology 1997;203:779-83. [PubMed]
- Mortelé KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001;21:895-910. [PubMed]
- Nishio J, Iwasaki H, Sakashita N, et al. Undifferentiated (embryonal) sarcoma of the liver in middle-aged adults: smooth muscle differentiation determined by immunohistochemistry and electron microscopy. Hum Pathol 2003;34:246-52. [PubMed]
- Varol U, Karaca B, Muslu U, et al. Undifferentiated embryonal sarcoma of the liver in an adult patient: case report. Turk J Gastroenterol 2012;23:279-83. [PubMed]
- Li XW, Gong SJ, Song WH, et al. Undifferentiated liver embryonal sarcoma in adults: a report of four cases and literature review. World J Gastroenterol 2010;16:4725-32. [PubMed]
- Ismail H, Dembowska-Bagińska B, Broniszczak D, et al. Treatment of undifferentiated embryonal sarcoma of the liver in children--single center experience. J Pediatr Surg 2013;48:2202-6. [PubMed]


