How to cope with a temporarily aborted transplant program: solutions for a prolonged waiting period
Introduction
Our university hospital used to be one of the biggest cardiac transplant centres in the lowlands (535 heart transplantations since 1981). Due to budgetary problems followed by financial restrictions its successful transplant program came to a complete standstill. The program was gradually restarted as of 2011. Henceforth waiting-times for newly listed transplant patients increased importantly compared to the average waiting-time of 10 months during the nineties. Since the advent of continuous flow LVAD devices an important improvement in survival has been reported in patients on the waiting-list (1). Because of the increased waiting-times a majority of our patients were in desperate need of a bridge-to-transplant device. We reviewed our early clinical results of assisted and non-assisted patients in this peculiar heart failure program over the past 4 years.
Patients and methods
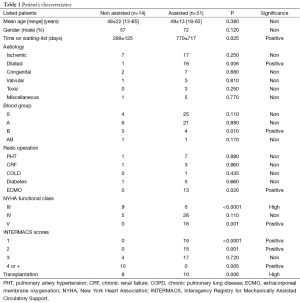
Since March 2011 until February 2015, 65 patients (mean age 48±23 years) were listed on the heart transplant list. One patient died on the waiting-list within the year. Eight patients (11%) of whom three in high emergency were transplanted without any form of mechanical assistance (average time on the waiting-list 320±267 days). Fifty-one patients required a left ventricular assist device (LVAD) Heartware (Heartware Inc., Miami Lakes, FL, USA) as a bridge-to-transplant due to terminal heart failure while despite optimal medical treatment and multiple dobutamine-cures (average time on the waiting-list before implantation of the LVAD 195±441 days). Henceforth only five patients remain on the waiting list without any mechanical support (time on the waiting-list 105±44 days). Patients in need of an LVAD were severely disabled by their disease all being in New York Heart Association (NYHA) functional class ≥IV; including nineteen crash-to-burn patients in NYHA class V who had been intubated and were on extracorporeal membrane oxygenation (ECMO) (n=16) or another temporary support (n=3). Risk stratification by Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) scores was calculated confirming the higher risk profile of the assisted patients compared to the non-assisted patients. Patient demographics of the two groups are listed in Table 1.
Full table
Follow-up
All patients were entered into a homemade database approved by the institution’s Ethical Committee. According to federal law, all causes of death (whether in-hospital or out-hospital) must be officially investigated and registered by a physician. The registry is open to access by certified physicians, but autopsies are only performed in-hospital on demand. After discharge, all surviving patients were asked to report at the institution at 4 weeks, and bi-monthly thereafter. One month after the initial implantation patients are entered in a cardiac revalidation program. Before any clinical examination, the patients were interrogated by staff members regarding their general health, medication and adverse events. The policy at the authors’ institution is to maintain LVAD patients on short-acting coumadin (acenocoumarol; half-life of 6-24 h), with an INR between 2-3 and aspirin 80 mg. All echocardiographic data were recorded during the first postoperative week and at further bi-annual follow up examinations by the same group of cardiologists. When patients were temporarily unable to attend the facility, the clinical, echocardiographic and laboratory data were acquired by mail, fax and/or phone from the referring general practitioners, specialists, or cardiologists.
Statistical analysis
Variables entered into the risk factor analysis included: age and time on the waiting list as continuous variables; and as categorical variables: age over 60 years; gender; Landsteiner blood group; etiology; redo operations; outside referral, the presence or absence of rhythm disturbances; preoperative NYHA class; INTERMACS scores; pre-op ECMO; LVAD support time; chronic renal failure (CRF); pulmonary artery hypertension (PHT); right heart failure; chronic pulmonary lung disease (COLD).
Continuous variables were expressed as the mean ± SD unless otherwise indicated. Kolmogorov-Smirnov test was used for normal distribution testing. Student’s t-test was used for parametric data, and Wilcoxon rank-sum test for non-parametric data. Post-hoc tests with Bonferroni correction were used in categorical variables with multiple levels to counteract the problem of multiple comparisons. The survival or event-free survival was analyzed using the Kaplan-Meier product-limit estimation. Univariate analysis of survival curves was performed using the log rank test (Mantel-Cox), while multivariable analysis of survival was performed using the Cox proportional hazards model. Only significant univariate variables were entered into a backward stepwise regression model. The level of significance was set at P<0.1 (SPSS 21 statistical program; SPSS Inc., Chicago, Illinois, USA).
Results
Mortality
Early deaths (90-days mortality)
Non-assisted patients; four elderly transplant patients (mean age 62±3, range 57-65 years) died of early graft failure (in all cases donor age was above 50 years). Six assisted patients, all operated in an emergency situation while on ECMO or temporary support, died within 90 days. The causes of death were: multiple organ failure and right heart failure in two patients each; respiratory insufficiency; and an irreversible cardiogenic shock in a uni-ventricular paediatric heart patient.
Late deaths
Non-assisted patients; one patient died within the year on the waiting-list of cardiac failure while one transplant patient died of acute rejection the first year. Of the assisted patients; one non-compliant patient succumbed at home to pneumonia and generalized sepsis without proper treatment 3 months after discharge from the hospital; one patient died of a traumatic brain hemorrhage 4 months post-op; one patient decided to end his treatment by his own free will 7 months after implantation; while two patients died of pulmonary hypertension and concomitant right heart failure (average support duration of 19±2 months).
Follow-up
All surviving patients have been discharged of the hospital (average length of stay 23±90 days). The follow-up is 100% complete with a total of 113 patient/years and an average of 15±6 months.
Transplanted patients
Four transplant patients without any previous form of assistance are alive and well as of this date. Since the restart of the program ten LVAD patients have been successfully transplanted [mean time on the waiting-list 770±717 days, mean LVAD support time was 367±254 days (range, 143-818 days)]. All patients are well except for one patient with a severe immune-mismatching struggling with recurred rejections treated by plasmapheresis and high doses of corticoids. Also included in the series are two previously transplanted patients who necessitated a LVAD implant because of graft failure (one early, one late) still on immune-suppression therapy.
Assisted patients
As of his date 30 LVAD patients remain on the waiting-list [mean waiting-time 627±380 days, mean LVAD support time 453±378 days (range, 1-1,207 days)], of whom twelve resumed their professional or scholar activities. Two hyper-immunized patients approach 4 years of waiting-time. In one patient the LVAD was removed after 19 months since the left ventricle had completely recovered from an ischemic cardiomyopathy once the 52-year-old recipient decided to stop her heavy smoking and drugs habits. One pump needed to be replaced 31 months after the initial implantation in a patient with a severe coagulopathy and repeated pump thromboses. One 30-year-old male quit his drug-abuse and entered a weaning phase ½ after his initial implantation and is forecasted to be explanted within months.
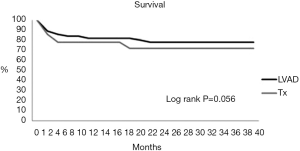
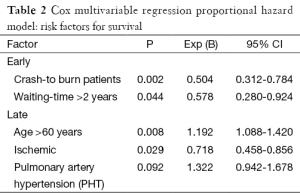
Survival at 1 and 3 years was respectively 78 (72%) and 83 (78%) for transplanted and assisted patients (Mantel-Cox log-rank P=0.056) (Figure 1). Cox multivariable regression analysis identified INTERMACS-1 patients (P=0.002) and waiting-times over 2 years (P=0.044) as risk factors for early death, while age above 60 (P=0.008), ischemic cardiomyopathy (P=0.029) and PHT (P=0.092) were risk factors for survival (Table 2).
Full table
Adverse events in assisted patients
Right heart failure
Seven episodes of right heart failure were recorded (five early, two late). Two patients necessitated an additional RVAD implantation, both died within 1 week. Three patients were treated early-on by vasodilatation en nitric oxide administration to be weaned off successfully within the first week. As mentioned before two patients would eventually succumb to their right heart failure 1 and ½ year after their initial LVAD implantation.
Pump thrombosis
Nine incidences of pump thrombosis were recorded in five different patients. One patient experienced five pump thromboses prior to his successful pump exchange despite being on an upgraded anti-coagulation and anti-platelet regime leading to a cerebral haemorrhage luckily without neurologic consequences. An examination of the explanted pump revealed an organized white thrombus at the bottom of the impeller. Except for the last episode (out of five) in the fore-mentioned patient with a severe coagulopathy all were successfully treated by thrombolysis. Abstraction made of the latter patient with the severe coagulation disorder, the incidences in the other four patients occurred under sub-therapeutic INR values.
Thromboembolism
Two episodes of ischemic stroke were recorded; both patients were well below target INR values of 2-3. Recovery was complete once their anti-coagulation regime was optimized.
Haemorrhage
The only fatal cerebral bleeding was traumatic of origin. Two episodes of cerebral bleeding occurred after thrombolysis, only one resulted in a permanent disability (hemi-anopsy of the left eye); another episode was due to mycotic emboli in the occipital sphere caused by a neglected dental abcedation however remained without neurological sequel. Two young female patients experienced severe vaginal bleeding caused by an intrauterine device (IUD) and needed surgical removal of the IUD and curettage. One patient diagnosed with haemorrhagic recto-colitis requires recurrent blood transfusions.
Overall costs and reimbursement
Total hospital costs of a transplant patient are calculated at 65,000 Euro of which 62,500 are fully reimbursed and 2,500 Euro are extra-charges for the patient. A total of 100,000 Euro is charged for a LVAD patient, including the device cost at 67,000 Euro. This amount is totally reimbursed with on top an extra 15,000 Euro charged by the company for the patient’s kit to the hospital. The hospital receives 900 Euro monthly the first year, and 450 Euro the second year for each surviving LVAD patient. In our country total artificial heart implantation is not reimbursed nor can be charged to the patient so the 250,000 Euro overall costs are an unbearable burden for our deficit-stricken department.
Comment
In our country some 3,000 patients per year are in need of their heart failure treatment. Although every year approximately 100 of those patients are listed for transplantation, the number of annual heart transplantations has decreased from 160 to 70 over 10-year time due to an increasing donor shortage. As a consequence, waiting-time on the transplant list rose to an average beyond 12 months for the whole country. Our situation is even more peculiar. Our transplant program used to be one of the biggest in the Lowlands (535 heart transplantations since the start in 1981) but due to financial restrictions it came to a complete standstill. It was gradually restarted beginning of 2011. Henceforth waiting-time for all newly listed patients as of 2011 increased dramatically to an average beyond 2 years. Since the advent of the third generation LVAD devices an important improvement in survival has been reported in patient on the waiting-list (2,3). Because of a prolonged waiting-time the majority of our patients (75%) were in desperate need of a bridge-to-transplant device. This resulted in the fact that over the last years we implanted far more devices than we transplanted, getting an ever longer waiting-list. Risk factors for early death of our listed patients were; INTERMACS-1 risk stratification profile and waiting-times over 2 years. Although all our early deaths in the LVAD group were crash-and-burn INTERMACS-1 patients, 13 others survived (68%) the initial period and are doing well including the ones successfully transplanted. This stands in contrast with the findings of Dell’Aquillo and co-workers who found no significant improvement in survival in INTERMACS-1,2 patients (4). Patients over two years on the waiting-list with a clear difference in their urgency status expressed a poor early survival in our series contrary to the findings of the Zurich group (5). However if a patient is in urgent need of circulatory support, the key to success is prompt instauration of an ECMO device (n=13) without delaying conversion to a proper LVAD since prolonged ECMO support is considered a risk factor for survival because of right ventricular failure. Veno-arterial ECMO provides a satisfactory peri-operative right-heart support in patients with pre-operative biventricular failure undergoing LVAD implantations allowing time for the already compromised right ventricle to get attuned to the increasing preload (6). Our results corroborate with the findings of the ReVOLVE study with the sole difference that our mean LVAD support time is 770 days compared to 363 days, thus double the time (7). The high incidence of early graft failure (22%) wasn’t due to the use of LVAD devices in our settings since none of the transplant patients was lost after LVAD explantation. It was the result of a rather reluctant acceptance of older, less optimal, donors for acceptors who became unstable. Younger donor age was an independent predictor of longer graft survival in the series of Maltais and co-workers (8). Therefore we are not inclined to increase the donor’s nor acceptor’s age on our list contrary to proposition of other centres (9). Right ventricle failure remains an issue. Elegantly demonstrated by Morgan and colleagues, LVAD support significantly decreased right filling pressures with concomitant improvement in RV function in patients without pulmonary hypertension or tricuspid insufficiency (10). However LVAD implantation complicated by early right ventricle failure has a poor prognosis. Since the average time on the waiting-list of our patients has increased to over 24 months some succumbed to pulmonary hypertension and concomitant right failure during their waiting-period even after RVAD implantation. We weren’t able to match the excellent results by Kutty and colleagues in patients with secondary hypertension (11). The difference might lie in the definition of PHT which we consider to have a resistance above 5 wood units (WU) while in the series of the Harefield group the average resistance in their PHT group was calculated 5.1±1.54 WU. A second LVAD can also be used as RVAD, however it is difficult to synchronize the pulmonary and the systemic flow thus keeping the patients hospital-bound (12). Pump thrombosis was a lesser issue except for patients with erratic INR values in our series (n=4). We could treat all patients by peripheral thrombolysis making abstraction of the last try of our patient with the severe coagulopathy (13). The latter had an organized white thrombus at the bottom of the centrifugal rotor rendering even intra-cardiac thrombolysis unsuccessful (14). Consequently pump exchange was performed and this patient awaits at home 1 year post-reoperation and is actually listed for over 1,000 days (15). The sole explantation because of full recovery of the left ventricular function was done in a female patient who changed drastically her life-style while another young male patient who quit completely his drug habits 1 and ½ after implantation seems to recover sufficiently to become an explant candidate over time. These findings of 2-4% of recovery are in accordance with other previous large series (16). Improvements in quality of life after LVAD have moved us into a new era of mechanical circulatory support for advanced heart failure. Nearly half of our LVAD patient retook their professional or scholar activities just as transplant patients do. We need to define the symptomatic ambulatory patients for whom co-morbidities and particular right ventricular dysfunction do not preclude good quality survival with LVADs. The additional hospital costs for transplantation and assist devices are equivalent for both type of patients but once over a 2-year waiting-time, additional costs for each LVAD patient will rise to over 10,000 Euro a year which in our settings of financial restriction will become a huge financial burden to the hospital contrary to findings of cost-effectiveness and savings of LVAD programs in other European health systems (17,18).
Conclusions
In our settings, a steep increase in LVAD implantation served to salvage patients for whom transplantation became jeopardized due to an ever increasing waiting-time. Henceforth circulatory LVAD support could even be considered as primary therapy. However at this time, there is not sufficient evidence to extend LVAD utilization to sicker patients or older patients. As long as reimbursement doesn’t match the real costs of this type of support, it remains one of the assets along transplantation and others within the framework of overall heart failure management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dell’Aquila AM, Schneider SR, Schlarb D, et al. Initial clinical experience with the HeartWare left ventricular assist system: a single-center report. Ann Thorac Surg 2013;95:170-7. [PubMed]
- Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012;125:3191-200. [PubMed]
- John R, Naka Y, Smedira NG, et al. Continuous flow left ventricular assist device outcomes in commercial use compared with the prior clinical trial. Ann Thorac Surg 2011;92:1406-13; discussion 1413. [PubMed]
- Dell’Aquila AM, Schneider SR, Stypmann J, et al. Survival results after implantation of intrapericardial third-generation centrifugal assist device: an INTERMACS-matched comparison analysis. Artif Organs 2014;38:383-90. [PubMed]
- Reser D, Fröhlich GM, Seifert B, et al. The impact of pretransplantation urgency status and the presence of a ventricular assist device on outcome after heart transplantation. Transplant Proc 2014;46:1463-8. [PubMed]
- Scherer M, Sirat AS, Moritz A, et al. Extracorporeal membrane oxygenation as perioperative right ventricular support in patients with biventricular failure undergoing left ventricular assist device implantation. Eur J Cardiothorac Surg 2011;39:939-44; discussion 944. [PubMed]
- Wu L, Weng YG, Dong NG, et al. Outcomes of HeartWare Ventricular Assist System support in 141 patients: a single-centre experience. Eur J Cardiothorac Surg 2013;44:139-45. [PubMed]
- Maltais S, Jaik NP, Feurer ID, et al. Mechanical circulatory support and heart transplantation: donor and recipient factors influencing graft survival. Ann Thorac Surg 2013;96:1252-8. [PubMed]
- Özalp F, Bhagra S, Bhagra C, et al. Four-year outcomes with third-generation centrifugal left ventricular assist devices in an era of restricted transplantation. Eur J Cardiothorac Surg 2014;46:e35-40. [PubMed]
- Morgan JA, Paone G, Nemeh HW, et al. Impact of continuous-flow left ventricular assist device support on right ventricular function. J Heart Lung Transplant 2013;32:398-403. [PubMed]
- Kutty RS, Parameshwar J, Lewis C, et al. Use of centrifugal left ventricular assist device as a bridge to candidacy in severe heart failure with secondary pulmonary hypertension. Eur J Cardiothorac Surg 2013;43:1237-42. [PubMed]
- Strueber M, Meyer AL, Malehsa D, et al. Successful use of the HeartWare HVAD rotary blood pump for biventricular support. J Thorac Cardiovasc Surg 2010;140:936-7. [PubMed]
- Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23-34. [PubMed]
- Kamouh A, John R, Eckman P. Successful treatment of early thrombosis of HeartWare left ventricular assist device with intraventricular thrombolytics. Ann Thorac Surg 2012;94:281-3. [PubMed]
- Stulak JM, Cowger J, Haft JW, et al. Device exchange after primary left ventricular assist device implantation: indications and outcomes. Ann Thorac Surg 2013;95:1262-7; discussion 1267-8. [PubMed]
- Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675-83. [PubMed]
- Pulikottil-Jacob R, Suri G, Connock M, et al. Comparative cost-effectiveness of the HeartWare versus HeartMate II left ventricular assist devices used in the United Kingdom National Health Service bridge-to-transplant program for patients with heart failure. J Heart Lung Transplant 2014;33:350-8. [PubMed]
- Mishra V, Fiane AE, Geiran O, et al. Hospital costs fell as numbers of LVADs were increasing: experiences from Oslo University Hospital. J Cardiothorac Surg 2012;7:76. [PubMed]