Survival following reirradiation using intensity-modulated radiation therapy with temozolomide in selected patients with recurrent high grade gliomas
Introduction
High grade gliomas are the most frequent primary central nervous system tumors in adults with an incidence of 6.03 per 100,000 adults per year and often recur (at least 70%) after initial treatment (1,2). Despite advances in imaging, anesthesia and surgical techniques, the prognosis of malignant gliomas treated by surgical resection alone is poor and median survival 4-6 months (3), with adding radiotherapy (RT) extending median survival to 8-9 months. Adding temozolomide extends median survival to 15 months for glioblastomas and 2-5 years for anaplastic gliomas (AG) (4). Despite all the treatment options, almost patients with high grade gliomas [grade III anaplastic astrocytoma (AA), grade IV glioblastoma multiforme (GBM)] and anaplastic histologic features tend to recurrence and usually have an extremely poor prognosis (5,6).
Most data suggest that, to stabilization of neurologic symptoms and additional survival time, re-treatment options can be applied for selected patients. There are several therapeutic options for the salvage treatment of patients after initial RT like as, re-resection, reirradiation (re-RT) or systemic agents with chemotherapy for recurrent gliomas. Despite so many treatment options, there is no standard treatment for recurrent gliomas. The prognosis is very poor and patients’ survival without treatment is a few months (7,8). However, after local relapse of gliomas, re-RT has been shown to be of value (9).
For re-RT of high grade gliomas, several RT techniques have been used to improve the therapeutic ratio including 3D-conformal RT, intensity-modulated radiation therapy (IMRT), brachytherapy, stereotactic radiosurgery (SRS) or stereotactic beam RT (SBRT) (9-13). However, radiation toxicity is very important in re-RT and various factors including dose per fraction, total cumulative radiation dose, fractionation scheme, time interval between first and second course RT, irradiated brain volume may influence brain tolerance for re-RT (10,14).
Temozolomide is an effective systemic agent for recurrent gliomas and is used concurrently with RT, although there is no consensus on an optimal regimen for recurrent disease (15). Major adverse effect of temozolomide is myelotoxicity, especially thrombocytopenia and neutropenia (16-18).
The aim of this retrospective study is to offer survival following re-RT using IMRT with temozolomide in patients with recurrent high grade gliomas.
Patients and methods
The design of the present study was approved by the Ethical Committee and Institutional Review Board of Necmettin Erbakan University Faculty of Medicine, where the study was conducted.
We identified 21 adult patients with recurrent primary brain tumor who were reirradiated with IMRT at the time of tumor recurrence or progression in Necmettin Erbakan University, department of radiation oncology between June 2010 and June 2014. Tumor recurrence was shown by gadolinium-enhanced magnetic resonance imaging (MRI) and diagnosis was established by pathology review in all cases.
Using the patients charts and electronic medical records with blinding to patients’ date of recurrence, last follow-up and death, the following data was obtained: sex, age, recurrent tumor pathology, location and size, extent of surgery, RT dose, biological effective dose (BED), whole brain RT dose, Karnofsky performance status (KPS) before the second course RT, time between RT courses, response to re-RT, use of chemotherapy, toxic effects of treatment and survival time.
Patients were evaluated after treatment for response as described in Table 1.
Study design
Inclusion criteria for re-RT were: (I) recurrent or progressive primary high grade brain tumor at the primary site; (II) measurable lesion on MRI; (III) ages between 18-70 years old; (IV) KPS ≥50; (V) an initial radiation dose between 30-60 Gy; (VI) an interval period between two RT course of at least 9 months. Patients were also required to have adequate hematological [hemoglobin >9 mg/dL, white blood cell (WBC) >4,000/mm3, absolute neutrophil count (ANC) ≥2,000/mm3, platelets ≥100,000/mm3], liver and renal functions (creatine ≤1.5 mg/dL). Patients with other solid organ metastases were excluded from the study.
Radiation therapy simulation and target volume definition
All patients were informed about the potential risks and benefits of radiation therapy and their informed consents were obtained. Simulation was performed using a clinical CT simulator. For simulation all patients were immobilized using a commercially available thermoplastic mask, supine with neutral neck position. CT image data was reconstructed in 3 mm slice thickness and then compared with preoperative MRI. The gross tumor volume (GTV) was defined as the enhancing lesion on T1 post-gadolinium and T2 FLAIR MRI sequences. The clinical target volume (CTV) included the GTV with a 2 cm expansion. Planning target volume (PTV) was delineated as the CTV plus 5 mm concentric expansion. The PTV was reduced in areas near organ at risks (OARs). These OARs included the brainstem, pituitary gland, cochlea, optic chiasm, optic nerves and lenses. All doses of OARs were in normally ranges.
Treatment planning
Twenty-one patients were treated with IMRT as prescribed to the International Commission of Radiological Units and Measurements reference point using a single phase IMRT technique and given temozolamide at dose of 75 mg/m2/day concomitant with RT. All patients underwent inverse planning on the EclipseTM treatment planning system (Varian Medical Systems, Palo Alto, CA).
Follow-up
Baseline physical and neurological examination and MRI were performed before re-RT. During the treatment patient were followed once weekly for new or treatment related complications, CBC, blood chemistry test and physical examination. Toxic effects were graded according to RTOG-Acute Radiation Morbidity Scoring Criteria. Clinical and radiological responses were evaluated every 3 months with clinical examination and MRI images.
Statistical analysis
Statistical analyses were performed with SPSS version 18.0.1 (SPSS Inc., Chicago, IL, USA) using Cox regression analyses (stepwise backwards, P in 0.05, P out 0.1), Kaplan-Meier method and log-rank test. Statistical significance was defined as P<0.05.
Results
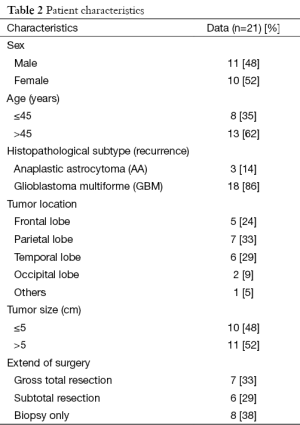
Between June 2010 and December 2014, 21 patients with recurrent high grade gliomas were treated with IMRT at the time of tumor progression or recurrence. The median age at the start of re-RT was 48 years (range, 19-67 years). Recurrent tumor size was median 5.5 cm (range, 2-9 cm). Patient characteristics were presented in Table 2.
Full table
At the time of first diagnosis, 11 (52%) patients were AA and 10 (48%) patients were GBM. Three (14%) patients were AA and 18 (86%) patients were GBM at the time of tumor progression or recurrence. Increasing grade existed at 8 (38%) patients in recurrence.
Eighteen patients presented by localized recurrence, three patients presented with diffuse recurrence. Median RT dose was 54 Gy (range, 25-60 Gy) for recurrent lesions. The BED cumulative was median 116 Gy (range, 105-150 Gy) and whole brain RT dose was median 28.7 Gy (range, 12.4-48.7 Gy). Before the re-RT, median KPS was 70 (range, 50-100). The time interval between two courses RT was median 39.3 months (range, 9.6-140.8 months). Out of 21 patients, 17 patients (81%) received temozolomide with re-RT.
The response was evaluated by MRI that was done 2 months after re-RT. Five patients (24%) achieved complete response (CR), 6 patients (29%) achieved partial response (PR). Stable disease (SD) was observed in 10 patients (47%). Disease progression (DP) was not observed.
The treatment protocol was well tolerated with only mild side effects. In 2 patients (10%) grade 1 neutropenia, in 2 patients (10%) grade 2 thrombocytopenia and in 3 patients (14%) grade 1 leucopenia was observed. Grade 1-2 toxicity in the form of nausea and vomiting occurred in 3 patients (14%). No grade 3 or 4 toxicity was recorded.
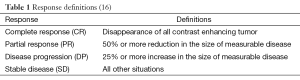
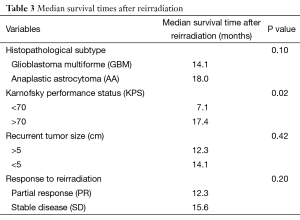
Median follow-up time from diagnosis was 41.4 months (range, 16.6-145.4 months). Median fallow-up time from re-RT was 12.3 months (range, 2-27.6 months). Median time to recurrence was 39.3 months (range, 9.6-140.8 months). Median survival after re-RT was 18 months for AA, 14.1 months for GBM (range, 11-17.2 months) (P=0.1) and was shown in Figure 1.
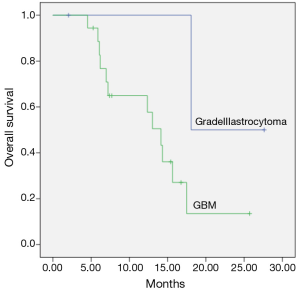
The median survival was 7.1 months for patients with KPS <70 before re-RT and 17.4 months for KPS >70 (P=0.02) and was shown in Figure 2.
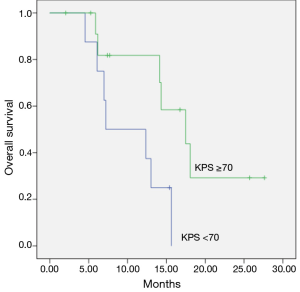
The median survival after re-RT was 12.3 months for patients with recurrent tumor size >5 cm and 14.1 months for <5 cm (P=0.42).
All patients who were CR to re-RT are alive, median survival for patients with PR was median 12.3 months (range, 6.7-13.8 months) and 15.6 months (range, 8.4-16.2 months) for patients with SD (P=0.2). Median survival times after re-RT was shown in Table 3.
Full table
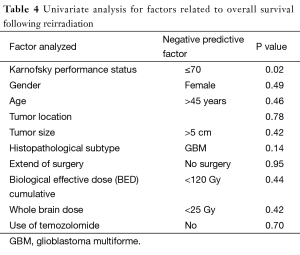
Univariate analysis for factors related to overall survival following re-RT showed that KPS ≤70 (P=0.02) and histopathological subtype of GBM (P=0.1) were negative predictive factors and all results was shown in Table 4.
Full table
Discussion
Despite the use of many effective treatment options in malign brain tumors, recurrence is very common in these patients and the optimum management for recurrent brain tumors has not been identified. Recurrent brain tumors are aggressive and incurable diseases. Reexisicion may often be difficult due to the patient’s medical condition and the potential for further neurological risk. RT with different re-RT techniques including 3-dimensional conformal radiation therapy (3D-CRT), IMRT, stereotactic RT, radiosurgery and brachytherapy is proposed as a therapeutic option in recurrent gliomas. However, prognosis is usually short (9,19-22). Most reports of primary GBM treated with IMRT, IMRT is available to deliver a high dose of radiation to the tumor with relative preservation of surrounding tissues to achieve optimal tumor coverage with minimal toxicity (23). In this study, we presented survival outcomes of recurrent high grade gliomas patient treated with IMRT and temozolomide in our center.
The prognosis of patients with malign brain tumors is poor and median survival of grade III AA patients is approximately 3 years and for GBM patients is 1 year (24).
Survival following IMRT for recurrent brain tumors have been explored (9,23,25). Fuller et al. reported the treatment results of 33 primary and 9 recurrent GBM patients who were treated with IMRT alone (72%) or as a boost (28%) after 3D-CRT. Twenty two patients (58%) had received chemo-radiotherapy. The median total radiation dose for all patients was 60 Gy with a range from 30.6 to 74 Gy. Median survival was 8.7 months and no survival difference was observed in IMRT boost vs. IMRT-only groups (25). Voynov et al. reported 10 patients with recurrent malignant gliomas (1 patient was low grade, 4 patients were AA, 4 patients were GBM and 1 patient was metastatic brain tumor) that were treated with IMRT. Before recurrence, all patients had been treated with external beam radiation therapy (median 59.7 Gy) and all recurrences were confirmed by a subtotal resection or radiological imaging. Re-RT was delivered in daily fractions of 5 Gy, to a total median dose of 30 Gy at the time of recurrence. Median overall survival time was 10.1 months from the date of IMRT for recurrent tumors, with 1- and 2-year survival rates of 50% and 33.3%, respectively (23). In other study, Mohammed Osman was reported 29 grade III and IV recurrent high grade gliomas patients treated with 3D-CRT, 30-40 Gy for re-RT and temozolomide. About 20.6% of patient’s achieved CR or PR with a SD rate of 34.4% (20).
Ertas et al. (24) reported 42 patients with histologically proven malign glioma (30 GBM, 12 AA) and also treated with previous conventional fractioned RT underwent stereotactic RT for re-RT. The median follow up time was 30 months, median survival from re-RT was 8 months for GBM patients and 11 months for AA patients.
In our study, the median survival from the diagnosis was 41.4 months. After re-RT median survival was 14.1 months for GBM and 18 months for AA (P=0.1). Our results are similar with the other reports which were reported survival advantage for recurrent high grade gliomas with other RT techniques such as stereotactic RT (24,26).
Temozolomide is an imidazotetrazine agent and effective in primary high grade gliomas (14). The efficacy and safety of temozolomide in treatment of recurrent high grade gliomas has been confirmed in several clinical trials (4,17,18,27,28), although there is no consensus on an optimal regimen for recurrent disease. A standard 5-day temozolomide regimen (150-200 mg/m2 for 5 of 28 days), and alternatively continuous daily dosing at 40-50 mg/m2; 100-150 mg/m2 at 7 days on/7 days off; and 75-100 mg/m2 at 21 days on/7 days off regimes can be used in recurrent high grade gliomas concomitant with RT (29). In this study we used standard temozolomide regimen.
However, use of temozolomide concurrently RT may be associated with hematologic or gastrointestinal toxicity (15). In our study, 5 patients (34%) had myelosupression (grade 1-2) and 3 patients (14%) had emetogenic side effects, grade 3-4 toxicity was not recorded.
In this present study, our survival data indicates that re-RT with IMRT technique may be useful with no grade 3 or 4 toxicity in recurrent high grade gliomas treatment.
There are some limitations in our study. First limitation of our study is that patient number is small but this is a preliminary study and one center experience in 4 years. And also, this is a retrospective study, however based on the results of this one, further study can be scheduled with more patient numbers. Another limitation of this study was in assessing focal symptoms and relief rate after treatment. However, based on our follow-up notes and data, none of our patients suffered from severe headache, nausea, vomiting or defect of vision.
Conclusions
re-RT is one of the treatment options for recurrent high grade gliomas and IMRT can be an effective treatment modality for recurrent high grade brain tumors with only mild side effects. Although survival is better in patients with good performance status and in recurrent anaplastic tumors after re-RT, further studies with more patient numbers can help to determine the role of this treatment and analyse toxicity following re-RT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dolecek TA, Propp JM, Stroup NE, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol 2012;14 Suppl 5:v1-49. [PubMed]
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359:492-507. [PubMed]
- Pichlmeier U, Bink A, Schackert G, et al. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 2008;10:1025-34. [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [PubMed]
- Melissa LB, El-Zein R, Scheurer ME. Epidemiology of brain tumors, Chapter1. In: DeMonte F, Gilbert MR, Mahajan A, et al. editors. Tumors of the brain and spine. M.D. Anderson Cancer Care Series. Berlin: Springer Science Business Media; 2007.
- Gilbert MR. Advances in the treatment of primary brain tumors: dawn of a new era? Curr Oncol Rep 2006;8:45-9. [PubMed]
- Nieder C, Adam M, Molls M, et al. Therapeutic options for recurrent high-grade glioma in adult patients: recent advances. Crit Rev Oncol Hematol 2006;60:181-93. [PubMed]
- Butowski NA, Sneed PK, Chang SM. Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin Oncol 2006;24:1273-80. [PubMed]
- Amichetti M, Amelio D. A Review of the Role of Re-Irradiation in Recurrent High-Grade Glioma (HGG). Cancers (Basel) 2011;3:4061-89. [PubMed]
- Veninga T, Langendijk HA, Slotman BJ, et al. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol 2001;59:127-37. [PubMed]
- Hess KR, Wong ET, Jaeckle KA, et al. Response and progression in recurrent malignant glioma. Neuro Oncol 1999;1:282-8. [PubMed]
- Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev 2000;26:397-409. [PubMed]
- Nieder C, Wiedenmann N, Andratschke N, et al. Current status of angiogenesis inhibitors combined with radiation therapy. Cancer Treat Rev 2006;32:348-64. [PubMed]
- Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys 2008;70:1350-60. [PubMed]
- van den Bent MJ, Keime-Guibert F, Brandes AA, et al. Temozolomide chemotherapy in recurrent oligodendroglioma. Neurology 2001;57:340-2. [PubMed]
- van Vugt VA, Piccioni DE, Brown BD, et al. Retrospective analysis of safety and feasibility of a 3 days on/11 days off temozolomide dosing regimen in recurrent adult malignant gliomas. CNS Oncol 2014;3:257-65. [PubMed]
- Han SJ, Rolston JD, Molinaro AM, et al. Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro Oncol 2014;16:1255-62. [PubMed]
- Omuro A, Chan TA, Abrey LE, et al. Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro Oncol 2013;15:242-50. [PubMed]
- Hentschel SJ, Sawaya R. Optimizing outcomes with maximal surgical resection of malignant gliomas. Cancer Control 2003;10:109-14. [PubMed]
- Osman MA. Phase II trial of temozolomide and reirradiation using conformal 3D-radiotherapy in recurrent brain gliomas. Ann Transl Med 2014;2:44. [PubMed]
- Kirkpatrick JP, Sampson JH. Recurrent malignant gliomas. Semin Radiat Oncol 2014;24:289-98. [PubMed]
- Palmer JD, Siglin J, Yamoah K, et al. Re-resection for recurrent high-grade glioma in the setting of re-irradiation: more is not always better. J Neurooncol 2015;124:215-21. [PubMed]
- Voynov G, Kaufman S, Hong T, et al. Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin Oncol 2002;25:606-11. [PubMed]
- Ertas G, Ucer AR, Guney YY, et al. Survival Following Stereotactic Radiotherapy for Recurrent High Grade Gliomas. Int J Hematol Oncol 2014;24:233-8.
- Fuller CD, Choi M, Forthuber B, et al. Standard fractionation intensity modulated radiation therapy (IMRT) of primary and recurrent glioblastoma multiforme. Radiat Oncol 2007;2:26. [PubMed]
- Kong DS, Lee JI, Park K, et al. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer 2008;112:2046-51. [PubMed]
- Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol 2013;15:4-27. [PubMed]
- Norden AD, Lesser GJ, Drappatz J, et al. Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro Oncol 2013;15:930-5. [PubMed]
- Wei W, Chen X, Ma X, et al. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: a systematic review with meta-analysis. J Neurooncol 2015;125:339-49. [PubMed]