Arterial blood supply of hepatocellular carcinoma is associated with efficacy of sorafenib therapy
Introduction
Hepatocellular carcinoma (HCC) is a clinically common disease. As the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections increases worldwide, the incidence of HCC also gradually rise. HCC is now the third leading cause of cancer deaths (1). Only about 15% of HCC have a chance to be cured by surgery (e.g., liver resection or transplantation) or minimally invasive local treatment (e.g., radiofrequency ablation). For HCC at advanced stages, radical treatment is impossible and multidisciplinary treatment may be more feasible (2).
As a multiple kinase inhibitor, sorafenib can selectively inhibit the vascular endothelial growth factor 2/platelet-derived growth factor receptor pathway to prevent tumor angiogenesis and meanwhile suppress cell proliferation by targeting the mitogen-activated protein kinase signaling pathway. Two global phase III clinical trials [SHARP (3) and Asia-Pacific trial (4)] have demonstrated that sorafenib can improve the prognosis of patients with advanced HCC. Among studies focusing on the prognostic factors of HCC patients treated with sorafenib, many of them found that the prognosis of these patients may be associated with the computed tomography (CT) and magnetic resonance imaging (MRI) features (5-10). Hahn et al. (6) found that the area under the contrast concentration vs. time curve 90 s after contrast injection (IAUC90) and the volume transfer constant of contrast agent (Ktrans) are pharmacodynamic biomarkers for sorafenib. Hsu et al. (7) also found that, among the prognostic factors of HCC patients receiving sorafenib and tegafur/uracil treatment, Ktrans was closely correlated with tumor response. In an experimental prostate cancer study in mice, sorafenib significantly suppressed tumor perfusion, vascular growth, and CT-visible endothelial permeability region (5). Therefore, CT and MRI identified biomarkers that may be feasible for detecting the possible response to antiangiogenic therapy in HCC patients.
In our current study, we explored the predictive factors on the efficacy of sorafenib in treating HCC, with a particular focus on the relationship between imaging findings and efficacy.
Materials and methods
Materials
A total of 38 consecutive HCC patients who were treated with sorafenib from April 2009 to December 2010 were included in this study. The diagnosis of HCC with good blood supply depends on at least two imaging approaches and featured by the remarkably enhanced lesion density in the arterial phase and decreased density in the venous phase; or, one imaging mode shows characteristic liver cancer lesion, along with an α-fetoprotein (AFP) level of ≥400 ng/mL. In contrast, the confirmation of HCC with poor blood supply is highly dependent on molecular biological approaches and histopathology. The inclusion criteria also included an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1 and a Child-Pugh grade of A. The enrolled patients were orally administered with 400 mg sorafenib (Bayer Healthcare, 200 mg × 2 tablets; twice daily). HCC was graded according to Barcelona clinic liver cancer (BCLC) staging system (11). Based on the results of enhanced CT or MRI scans, the HCC patients were divided into rich blood supply group and poor blood supply group. The imaging diagnoses were performed by specialists with rich experiences (without referring to the clinical features of the patients). Lesions with a hyperenhancement area of ≥60% were defined as with good blood supply, and those with a hyperenhancement area of ≤40% as poor blood supply.
Methods
The follow-up data included the monthly laboratory test results (liver and kidney functions and AFP) and imaging findings (chest radiography, and abdominal CT or MRI). PET-CT was immediately performed if tumor metastasis was suspected. Transcatheter arterial chemoembolization (TACE) was performed if necessary. The adverse reactions following drug administration were monitored and properly managed.
Statistical analysis
The count data are presented using mean ± standard deviation, and their differences were compared using t-test. Measurement data were compared using Chi-square test or Fisher’s exact probability test, and a P value of <0.05 was regarded as statistically significant. HR and 95% CI were calculated using the logistic regression formula. Factors found to be statistically significant (P<0.05) during univariate analysis were further analyzed using Logistic multivariate regression analysis, so as to identify the relatively independent risk factors. Survivals were analyzed using the Kaplan-Meier estimator with log-rank testing. Prognostic analysis was performed using Cox regression model. The overall survival (OS) and tumor-free survival (TFS) were calculated from the initiation of treatment to death and from the initiation of treatment to the deadline for follow-up, respectively. The follow-up deadline was August 31, 2011.
Results
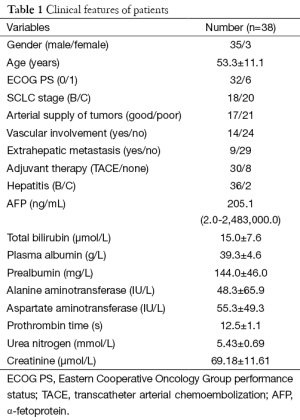
Among these 38 patients, there were 35 men and 3 women, with a mean age of (53.3±11.1) years. All of them had a history of hepatitis, among which hepatitis B accounted for 94.7%. The clinical data of these patients are summarized in Table 1. HCC with good blood supply was seen in 17 cases, and those with poor blood supply in 21 cases. Up to 30 patients (13 from the good blood supply group and 17 from poor blood supply group) received TACE during the follow-up.
Full table
Adverse reactions
During the sorafenib treatment, each patient had experienced at least one adverse event, among which hand/foot rashes and diarrhea were most common, whereas fatigue, alopecia, hypertension, and diabetes were relatively rare. The dose of sorafenib was tapered due to severe adverse reactions in 6 patients (3 due to severe diarrhea and 3 due to severe rashes). No drug withdrawal was noted during the follow-up.
Survival analysis
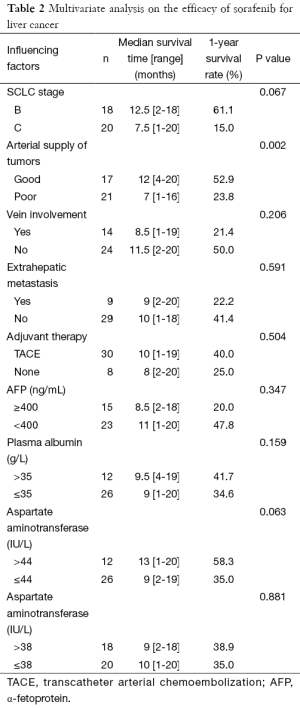
Up to 29 patients (76.3%) died during the follow-up, yielding a median survival time (MST) of 10.7 months (95% CI, 8.7-12.7) and a 1-year OS of 41%. The MST and 1-year OS in patients with tumors with good arterial supply were 12 months (range, 4-20 months) and 52.9%, respectively, compared with those of 7 months (range, 1-16 months) and 23.8% in patients with tumors with poor arterial supply (P=0.002). The MST and 1-year OS were better in patients with BCLC stage B HCC than those with BCLC stage C HCC, although the differences were not statistically significant.
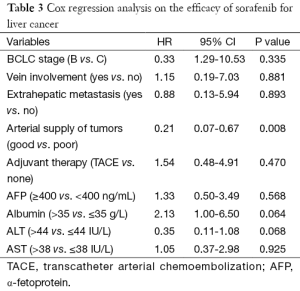
The results of multivariate analysis and Cox regression analysis are shown in Tables 2,3. It was found that the arterial blood supply of tumor is a prognostic factor for OS in HCC patients (HR =0.22; 95% CI, 0.07-0.67; P=0.008).
Full table
Full table
Discussion
As a tumor with good blood supply, HCC showed hyper-enhancement on CT or MRI in most cases; more specifically, it showed obvious hyper-enhancement during arterial phase, and such enhancement gradually decreases during the venous phase and disappears during the delayed phase (1). The arterial-phase enhancement is not obvious in only a small number of HCC cases.
In our current study, we found the prognosis of HCC patients treated with sorafenib was closely associated with the degree of arterial-phase enhancement, and the prognosis of patients with HCC with good blood supply was significantly better than that of patients with HCC with poor blood supply. Li et al. (12) and Ippolito et al. (13) found that CT could provide sufficient information on tumor-related vessels and thus can be a good tool for evaluating the degree of tumor vascularization. Blood supply from hepatic artery and portal vein can be relatively insufficient during the rapid growth of HCC; as a result, the new vessels continuously form around the tumor, leading to the formation of tumors with good blood supply (14,15). The degree of arterial-phase enhancement on CT or MRI may be an important marker of tumor vascularization. The newly formed vessels are extremely important during the development of tumors (16), whereas the vascular endothelial growth factor play a key role in regulating angiogenesis and endothelial cell proliferation (17). Many studies have found that the expression of vascular endothelial growth factor in HCC is closely related with imaging findings (18-21). Kwak et al. (21) found that the obvious arterial-phase enhancement in HCC was due to vascular endothelial growth factor over-expression, which further results in the constant increase of vascular permeability and the proliferation of vascular endothelial cells. In our current study, the degree of arterial-phase enhancement was positively correlated with the prognosis of HCC patients treated with sorafenib. Although it has been proposed that the vascular endothelial growth factor level may affect the efficacy of sorafenib in treating HCC, more studies are warranted to further demonstrate the relationship between the activation of vascular endothelial growth factor and the efficacy of sorafenib in treating HCC. Also, prospective randomized controlled trials are needed to clarify the relationship between the degree of HCC arterial-phase enhancement and the prognosis of HCC patients treated with sorafenib.
In summary, arterial blood supply is an independent predictor for survival in HCC patients treated with sorafenib, and patients with tumors with good arterial supply benefit more than those with tumors with poor arterial supply.
Acknowledgements
Funding: This work was supported by National Science and Technology Major Project Foundation (2008ZX10002-025) and Natural Science Foundation of Hubei Province (2013CFB477), China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinom. Lancet 2012;379:1245-55. [PubMed]
- El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134:1752-63. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Cyran CC, von Einem JC, Paprottka PM, et al. Dynamic contrast-enhanced computed tomography imaging biomarkers correlated with immunohistochemistry for monitoring the effects of sorafenib on experimental prostate carcinomas. Invest Radiol 2012;47:49-57. [PubMed]
- Hahn OM, Yang C, Medved M, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 2008;26:4572-8. [PubMed]
- Hsu CY, Shen YC, Yu CW, et al. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol 2011;55:858-65. [PubMed]
- Flaherty KT, Rosen MA, Heitjan DF, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 2008;7:496-501. [PubMed]
- Horger M, Lauer UM, Schraml C, et al. Early MRI response monitoring of patients with advanced hepatocellular carcinoma under treatment with the multikinase inhibitor sorafenib. BMC Cancer 2009;9:208. [PubMed]
- Lewin M, Fartoux L, Vignaud A, et al. The diffusion-weighted imaging perfusion fraction f is a potential marker of sorafenib treatment in advanced hepatocellular carcinoma: a pilot study. Eur Radiol 2011;21:281-90. [PubMed]
- Marrero JA. Staging systems for hepatocellular carcinoma: should we all use the BCLC system? J Hepatol 2006;44:630-2. [PubMed]
- Li JP, Zhao DL, Jiang HJ, et al. Assessment of tumor vascularization with functional computed tomography perfusion imaging in patients with cirrhotic liver disease. Hepatobiliary Pancreat Dis Int 2011;10:43-9. [PubMed]
- Ippolito D, Sironi S, Pozzi M, et al. Hepatocellular carcinoma in cirrhotic liver disease: functional computed tomography with perfusion imaging in the assessment of tumor vascularization. Acad Radiol 2008;15:919-27. [PubMed]
- Asayama Y, Yoshimitsu K, Nishihara Y, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol 2008;190:W28-34. [PubMed]
- Tajima T, Honda H, Taguchi K, et al. Sequential hemodynamic change in hepatocellular carcinoma and dysplastic nodules: CT angiography and pathologic correlation. AJR Am J Roentgenol 2002;178:885-97. [PubMed]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6. [PubMed]
- Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 1993;12:303-24. [PubMed]
- von Marschall Z, Cramer T, Höcker M, et al. Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 2001;48:87-96. [PubMed]
- Kanematsu M, Osada S, Amaoka N, et al. Expression of vascular endothelial growth factor in hepatocellular carcinoma and the surrounding liver: correlation with angiographically assisted CT. AJR Am J Roentgenol 2004;183:1585-93. [PubMed]
- Suzuki K, Hayashi N, Miyamoto Y, et al. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res 1996;56:3004-9. [PubMed]
- Kwak BK, Shim HJ, Park ES, et al. Hepatocellular carcinoma: correlation between vascular endothelial growth factor level and degree of enhancement by multiphase contrast-enhanced computed tomography. Invest Radiol 2001;36:487-92. [PubMed]


