Immune checkpoint inhibitors: therapeutic advances in melanoma
Introduction
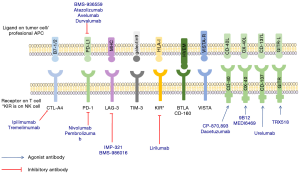
Cancer behaves like a “self” protein since the immune system is unable to recognize it cancer as a danger (1). Immune checkpoints are activated in immune cells to prevent inflammatory immunity developing against the “self”. Therefore, changing the balance of the immune system via checkpoint inhibition should allow it to be fully activated to detect and eliminate the cancer. The best known inhibitory receptors implicated in control of the immune response are cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), T-cell immunoglobulin domain and mucin domain-3 (TIM3), lymphocyte-activation gene 3 (LAG3), killer cell immunoglobulin-like receptor (KIR), glucocorticoid-induced tumor necrosis factor receptor (GITR) and V-domain immunoglobulin (Ig)-containing suppressor of T-cell activation (VISTA) (2-4) (Figure 1). Several therapeutic antibodies have been developed against these receptors but so far only the anti CTLA-4 antibody ipilimumab and the anti-PD-1 antibodies nivolumab and pembrolizumab have been approved for treatment of metastatic melanoma. These treatments have achieved long lasting responses and a 20-40% long survival rate. However, median progression free survival (PFS) and response rates are less impressive (5-7).
In this review, we summarize the results of the main studies performed with antibodies that target the immune checkpoint identified as very relevant to elicit immune tolerance to cancer, and describe other of the most promising checkpoint inhibitors that have been considered for new therapeutic development.
Anti CTLA-4 antibodies
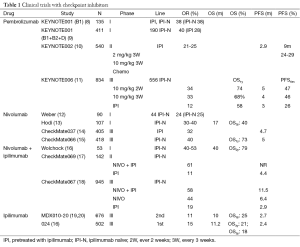
Two human mAbs, ipilimumab and tremelimumab, against CTLA-4 have entered clinical trials (Table 1) (Figure 1). However, only ipilimumab has been approved by the Food and Drug Administration (FDA) and European Medical Agency (EMA) for use in metastatic melanoma patients. These drugs have a specific toxicity profile where most adverse events (AEs) reported are immunologic in nature and require specific management in order to avoid severe toxicity. A second differential characteristic of these drugs is that special patterns of response can be observed; special response criteria has had to be developed that are currently applied to other immunotherapies (21).
Full table
Ipilimumab
Ipilimumab is a fully human IgG1 mAb approved for use in melanoma patients at a 3 mg/kg dose every 3 weeks for 4 doses. The first phase I trial was reported in 2002 and demonstrated a 11.7% response rate (22). Several subsequent phase II trials with different doses ranging from 0.3 to 10 mg/kg have shown a trend to greater responses with increasing doses of ipilimumab. However, this increase was paralleled by an increase in toxicity (23). Early phase II trials showed some very long lasting responses and survival (24-26). The first phase III trial was conducted in the second line setting and compared ipilimumab 3 mg/kg monotherapy with GP-100 vaccine or the combination of both (19). The trial resulted in a significant improvement of survival of 3.5 months for any of the arms containing ipilimumab. It was also noticeable that 2-year survival was 21.6% for the combined ipilimumab and GP-100 arms, 23.5% for ipilimumab alone and only 13.7% for GP-100 alone. With longer follow up the percentage of 20% long term survivors was maintained (20) and is considered a differential characteristic of this treatment (Table 2). The clinical trial of ipilimumab in first line compared the drug at a dose of 10 mg/kg for 4 doses and maintenance every 12 weeks plus DTIC vs. DTIC alone (27) (Table 3). Again, an improvement in survival (median 11.2 months) was noted for the ipilimumab arm and maintained with prolonged follow up, with 18.2% of ipilimumab treated patients surviving more than 5 years (27). These results in the metastatic setting have led to adjuvant therapy trials that are not yet mature in terms of overall survival (OS) but which are showing promising results in recurrence free survival of 26.1 months for ipilimumab treated patients and 17.1 for placebo treated (29). The question of what the optimal dose could be is still unresolved; the most notable differences are seen in toxicity that is higher at larger doses. However, there is no clear effect on long term survival. A phase III trial comparing the two doses has been concluded but not yet reported. Since ipilimumab is one of the standard treatments for metastatic melanoma patients, its role in several combinations with other immunotherapies, chemotherapy and targeted therapy is currently being studied (30).

Full table

Full table
Tremelimumab
Tremelimumab is an IgG2 human mAb which was also known as ticilimumab during early development. Phase I and II trials showed it was a safe drug with some activity and the optimal dose was defined as 15 mg/kg every 90 days (31). This dose was chosen because it was safer than the 10 mg/kg monthly with similar results in response, OS and disease free survival. Tremelimumab was compared as a single agent to dacarbazine or temozolomide in a phase III trial in patients with untreated unresectable or metastatic melanoma (32). The primary objective of this trial was OS, which was 11.8 months for the tremelimumab arm and 10.7 for the chemotherapy arm. Therefore, the trial was considered negative. Several explanations have been suggested for the differences between ipilimumab and tremelimumab, one being a possible different mechanism of action since tremelimumab is an IgG2 and lacks the antibody dependent cell cytotoxicity that is observed with ipilimumab, resulting in a depletion of regulatory T cells (Tregs) (33,34). Another possibility is that patients in the chemotherapy arms may have been switched to ipilimumab during the trial and therefore access to ipilimumab was expanded (32). It is notable that long term results with tremelimumab are not so different from ipilimumab: final results of the phase III trial in terms of 2- and 3-year survival are 26.4% and 20.7%, respectively (32). Also, patients from phase I and II trials have been assessed for long term survival, with Kaplan-Meier 5-year survival of 20% and 5-year survival of 16% (35). As a result interest in this drug is still active and currently combination trials of this drug with anti-PDL1 antibodies and other immunotherapies are being conducted (30).
Prediction of response to anti CTLA-4 antibodies
The most important challenge for CTLA-4 drugs is selecting those patients most likely to respond. Several patient characteristics, such as good performance status, low tumor burden, low LDH level and count or increase in count of lymphocytes with the treatment have been proposed as markers of response, however, none are powerful enough (36,37). Markers of inflammatory microenvironment have also been suggested for patients receiving ipilimumab (38) but these are still not standardized. Recently neo-antigens have been proposed as important for the efficacy of immunotherapies since the greater the differences between antigens in the tumor, the more likely it is that the tumor will be recognized by the immune system and that this response could be enhanced (39). Mutational load of patients with melanoma has been linked to benefit for ipilimumab and tremelimumab and a genetic signature has been identified for prediction of response in these patients ((40). The best way of identifying patients with a high possibility of response will probably be some combination these approaches. Identification of predictors of response is highly important since selected patients could receive just four doses of ipilimumab instead of the more complex anti-PD-1 treatment regimens.
Anti PD-1 antibodies
The PD-1 receptor is an inhibitory receptor engaged by its ligands PD-L1 (also known as B7-H1 or CD274) and PD-L2 (also known as B7-DC or CD273) (Figure 1). PD-1 is present in T activated cells, B cells, monocytes, and natural killer (NK) cells. PDL-1 is expressed in several cells, such as tumor cells and some epithelial cells, lymphoid cells, myeloid cells and antigen-presenting cells (APCs). PD-1 activity occurs primarily within the tumor microenvironment, more peripherally than CTLA-4 action (41,42). Like CTLA-4, PD-1 and its ligands PD-L1 and PD-L2 also affect TCR and inhibit functional T-cell signaling. PD-1 later becomes operative in T-cell response once inflammatory cytokines have been released. PD-1/PD-L1 interactions limit inflammation and inhibit CTL activity. PD-1 activation inhibits CD8+ cytotoxic T lymphocyte proliferation, survival and their effector function and can also induce apoptosis of TILs and promote differentiation of CD4+ T cells into Treg cells. PD-1 ligation by PD-L1 or PD-L2 recruits SHP2, resulting in membrane proximal decreases in TCR signaling by dephosphorylating the CD3-zeta chain. The anti PD-1 antibodies at most advanced stages of development in melanoma are nivolumab and pembrolizumab.
Pembrolizumab
Pembrolizumab (formerly known as lambrolizumab or MK-3475) is a humanized mAB that blocks the PD1 receptor in the T lymphocytes, thus releasing them from its inhibitory interaction with PD-L1 and PD-L2 and provoking an immune response against tumor cells. It is currently approved by the FDA for the treatment of advanced melanoma patients who have progressed to ipilimumab and, in the case of BRAF mutant melanoma patients, also to a BRAF/MEK inhibitor. Pembrolizumab has an accelerated grant priority review for approval in first line, both for BRAF mutant and wild type melanoma (43). The first clinical trial of pembrolizumab analyzed safety, response rate and PFS in 135 patients with advanced melanoma in an expansion cohort of the Keynote001 clinical trial, a large and complex “phase I” clinical trial with more than 650 melanoma patients treated (in addition to other tumors). In this first cohort, RECIST 1.1 overall response rate (ORR) was up to 44%, being 25% in the cohort of 2 mg/kg every 3 weeks, and 52% in the cohort of 10 mg/kg every 2 weeks. The median PFS in the whole cohort was 7 months (8). At the time of publication of this trial, BRAF inhibitors (for BRAF mutant melanoma) or ipilimumab (for both BRAF mutant and wild-type) was the standard of care for melanoma treatment. Thus, analyzing if pembrolizumab was active in those patients who had exhausted these treatments was of great importance. Two studies, one an expansion cohort of the KEYNOTE001, and the other a phase II clinical trial, KEYNOTE 002, explored this clinical scenario. In Keynote 001, 173 patients, who progressed to ipilimumab and, if BRAF mutant to a BRAF and/or MEK inhibitor, were randomized to receive pembrolizumab 2 mg/kg every 3 weeks or 10 mg/kg every 3 weeks, with ORR as primary endpoint (evaluable in 156 patients). ORR was 26% at both doses. The percentage of patients alive at 1 year was 58% and 63% for 2 and 10 mg groups respectively, this being the most striking finding of the trial since heavily pre-treated patients had been included. Of note, only 26 patients (17% of evaluable patients) were BRAF mutant (44), in contrast to the general epidemiology of BRAF mutation, usually positive in 50% of melanoma population. These could be explained by the fact that patients with BRAF mutant melanoma, usually progress with more aggressive disease that those wild type after ipilimumab. This study led to approval of pembrolizumab by the FDA for its current indication (Table 1).
The other study that explored the ipilimumab refractory scenario was a phase II clinical trial, KEYNOTE 002. In this study, 540 patients refractory to ipilimumab and to BRAF/MEK inhibitors (if BRAF mutant) were randomized to receive pembrolizumab 2 mg/kg [180] vs. pembrolizumab 10 mg/kg [181], both every 3 weeks, or physician’s choice chemotherapy [179]. In the second interim analysis, PFS was the primary end-point, and it was demonstrated that both pembrolizumab doses were superior to chemotherapy, with a hazard ratio (HR) of 0.57 for 2 mg/kg and 0.50 for 10 mg/kg vs. chemotherapy. Median PFS was around 5 months for the pembrolizumab 2 mg/kg group, 6 months for the pembrolizumab 10 mg/kg group, and 3 months for the chemotherapy group. OS data are still immature, but it is important to note that almost half the patients who progressed to chemotherapy crossed-over to pembrolizumab, something which should be taken into account for future analyses (10). From these two clinical studies we can conclude that pembrolizumab 2 mg/kg is the dose of choice, since 10 mg/kg is almost equivalent in terms of efficacy end-points.
Recently, a phase III clinical trial, KEYNOTE006, has compared pembrolizumab vs. ipilimumab, the previous standard of care in first and second line. This study included 834 patients, 2/3 treatment naive, irrespective of BRAF status. Patients were randomized to receive pembrolizumab 10 mg/kg every 2 weeks up to 2 years (279 patients), vs. 10 mg/kg every 3 weeks up to 2 years (277 patients), versus ipilimumab 3 mg/kg every 3 weeks for 4 cycles (278 patients). OS and PFS were co-primary end-points. Two interim analyses were pre-specified in order to detect an early effect on OS with pembrolizumab over ipilimumab. Median PFS was 5.5 months for pembrolizumab every 2 weeks, 4 months for pembrolizumab every 3 weeks and 2.8 months for ipilimumab, with a HR of 0.58. Median OS was not reached for any of the treatment groups. One-year estimates OS were 74% for patients receiving pembrolizumab every 2 weeks (HR =0.63), 68% for those receiving pembrolizumab every 3 weeks (HR =0.69), and 58.2% for those receiving ipilimumab. The study was early stopped since the OS results for pembrolizumab groups were superior to the ipilimumab group at the pre-specified interim analysis. Patients on ipilimumab were offered the opportunity to cross over to pembrolizumab. All stratification factors, including BRAF status, line of therapy and PD-L1 status (defined as positive with a >1% staining), benefited pembrolizumab in terms of PFS. However, in PD-L1 negative patients (18% of the global sample), HR for OS was 1.02 and 0.91 for both pembrolizumab groups (11). This clinical trials lead to consideration for priority approval by the FDA of pembrolizumab as first line treatment in advanced melanoma, independently of the BRAF status (Tables 1,4). However, in this study, patients who were BRAF mutant should have been treated with BRAF inhibitors if they had elevated lactate dehydrogenase levels and/or clinically significant tumor-related symptoms (11). As a result, around 35% of patients were BRAF mutant (again, in a lesser proportion than epidemiological data) and were clinically selected in first line due to better prognostic factors (11) than first line BRAF mutant patients in pivotal BRAF inhibitor studies (45). Another issue is the dose used in this clinical trial, 10 mg/kg, in contrast with the currently approved dose of 2 mg/kg. The same issue arose with the approval of ipilimumab at a dose of 3 mg/kg, which had never been used before FDA approval in a phase III clinical trial with treatment naïve patients, in contrast to the 10 mg/kg in combination with DTIC in first line (27). One of the multiple expansion cohorts of the KEYNOTE001 trial randomized 103 ipilimumab naïve patients to receive 2 mg/kg every 3 weeks vs. 10 mg/kg (46). If survival results are similar, as occurred in the ipilimumab pre-treated cohort of this trial (44) as well as in the KEYNOTE002 (10), 2 mg/kg could be extrapolated to the first line scenario.
Full table
Toxicity of pembrolizumab has been consistent in all clinical trials, being less frequent and less severe than toxicity related to chemotherapy (10) or with ipilimumab (11). In KEYNOTE-001 the incidence of grade ≥3 AE was 14% in the nonrandomized melanoma cohorts (n =135) (47) and 12% in the randomly assigned ipilimumab-refractory patients (n=173) (44). In KEYNOTE-006, the pembrolizumab arms had less toxicity compared with ipilimumab: grade ≥3 toxicity incidence was 13.3%, 10.1%, and 19.9% for the 10 mg/kg Q2W vs. 10 mg/kg Q3W doses of pembrolizumab and ipilimumab, respectively (11). The rate of drug discontinuation secondary to AEs for these groups was 4.0%, 6.9%, and 9.4%, respectively. Common AEs observed with pembrolizumab were fatigue, diarrhea, hyperthyroidism, hypothyroidism, rash, and pruritus; grade ≥3 colitis occurred in 1.4% and 2.5% and grade ≥3 hepatitis in 1.1% and 1.8% at the Q2W and Q3W schedules, respectively (11).
Predictive factors for pembrolizumab activity have focused on PD-L1 expression measured by immunohistochemistry (IHC). In a 195-patient training set derived from the melanoma population of KEYNOTE-001 and using a 1% cutoff to determine positivity, 71% of the 125 evaluable patients had PD-L1-positive tumors as assessed using a prototype IHC assay and the 22C3 antibody (48). PD-L1 positivity was associated with higher ORR by RECIST v1.1 (49% vs. 13%; P=0.0007) and longer PFS (median 11 vs. 3 months; HR =0.52; 95% CI, 0.32-0.86; P=0.0051), but no differences were observed in terms of OS (6-month OS was 91% in positive vs. 79% in negative PD-L1 tumors; P=0.3165) (48). In an independent validation set of 216 patients with melanoma in KEYNOTE-001, 82% of the 150 evaluable patients had PD-L1-positive tumors. Similar to the training set, PD-L1 positivity vs. PD-L1 negativity was associated with a higher ORR (36% vs. 4%; P=0.0022), longer PFS (HR =0.43; 95% CI, 0.27-0.69; P=0.0002), and longer OS (HR =0.33; 95% CI, 0.18-0.63; P=0.0042) (49). Although PD-L1 positivity is correlated with response to pembrolizumab in melanoma, given the response rates seen in patients with PD-L1-negative tumors and the high prevalence of PD-L1 positivity, it is unlikely that PD-L1 will be used as a selection or predictive marker for anti-PD-1 or anti-PD-L1 agents. Data from the KEYNOTE-006 trial confirm activity in PD-L1-negative tumors in terms of PFS but not OS (however, only 18% of tumors were PD-L1 negative, so the confidence interval (CI) was very large and no conclusions could be derived from this analysis) (11).
Other predictive models including PD-1+ density, PD-L1+ density, CD8+ infiltration and CD4+ density in the tumor and at the tumor margin, could be more accurate for identifying those patients with higher chances of response (50).
Recently it has been described the predictive value of an IFNγ immune gene signature developed using NanoString nCounter Gene expression assay. A immune score was calculated with a positive predictive value of 59% and a negative predictive value of 90% in a validation cohort of 62 patients from Keynote001 trial (51).
Nivolumab
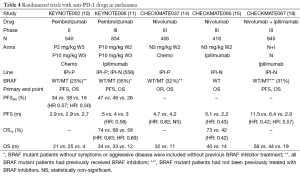
Nivolumab (BMS-936558) is a fully human immunoglobulin G4 (IgG4) mAb anti-PD-1 agent. The phase I trial showed evidence of antitumor activity and an acceptable safety profile (52,53). A total of 104 patients with melanoma were enrolled. Objective response rate between 19% and 41% was observed at doses ranging from 0.1 to 10 mg per kg, and stable disease lasting 24 weeks or more was observed in 6%. Grade 3 or 4 drug-related AEs occurred in 14% of patients, and there were three deaths due to pneumonitis. Another phase I trial with nivolumab at multiple doses with or without a multipeptide vaccine was conducted to evaluate safety and efficacy and to explore immune predictors of response. Ninety unresectable stage III or IV melanoma patients who were ipilimumab naive (34 patients) or who had experienced progression after ipilimumab (56 patients) were included. Nivolumab with vaccine was well tolerated and safe at all doses. The confirmed objective response rate for ipilimumab-naïve cohorts was 24%, with a disease control rate of 45%. In ipilimumab-refractory patients the objective response rate was 26%, with a disease control rate of 47% (12). OS and long-term safety data from the first phase I trial were published by Topalian et al. in 2014 (28). Median OS was 16.8 months, with 1- and 2-year survival rates of 62% and 43%, respectively. Objective response rate was 31% and median duration of response was 24 months in these patients. It was interesting that in those patients who discontinued therapy for reasons other than disease progression, the responses off-therapy were maintained during 16 weeks or longer. The most common AEs: fatigue (32%), rash (23%) and diarrhea (18%). Grade 3 or 4 toxicities appeared in 22% of patients. Most AE occurred within the first six months of therapy and there were no cumulative toxicities with prolonged drug exposure.
The efficacy and safety of nivolumab in patients pretreated with ipilimumab, or with ipilimumab and BRAF inhibitors, was evaluated in an open-label, phase III trial (CheckMate 037) (14) (Tables 1,2,4). Primary endpoint was objective response rate. A total of 405 patients were randomized 2:1 to nivolumab (272 patients) or chemotherapy (133 patients). Confirmed objective response rate was 31.7% (95% CI, 23.5-40.8) in the nivolumab group vs. 10.6% (95% CI, 3.5-23.1) in the ICC group. There were 8% of unconventional immune-related response (regression or stable disease after progression) in nivolumab arm. At the time of analysis, 87% of nivolumab responses were continuing on treatment without progression. There was no statistically significant difference in PFS: 4.7 months in nivolumab arm vs. 4.2 months in ICC group (HR =0.82; 95% CI, 0.32-2.05). AEs related to treatment were reported in 68% of nivolumab patients and 79% of ICC patients. Grade 3-4 AEs occurred in 9% of nivolumab treated patients vs. 31% in the ICC group. The most frequent AEs in the nivolumab arm were fatigue, pruritus and diarrhea. On the basis of these results, in 2014 the FDA approved nivolumab for metastatic melanoma patients who progressed after treatment with ipilimumab and a BRAF inhibitor, in BRAF mutated population.
A double-blind phase III trial was conducted to assess efficacy of nivolumab in previously untreated advanced BRAF wild type melanoma patients (CheckMate 066) (Tables 1,4). A total of 418 patients were randomized to receive nivolumab (3 mg/kg every 2 weeks and dacarbazine-matched placebo every 3 weeks) or dacarbazine (1,000 mg/m2 every 3 weeks and nivolumab-matched placebo every 2 weeks). The primary end point was OS. At 1 year, the OS rate was 72.9% in the nivolumab group vs. 42.1% in the dacarbazine group (HR =0.42; 99.79% CI, 0.25-0.73; P<0.001). The median PFS was 5.1 months in the nivolumab group vs. 2.2 months in the dacarbazine group (HR =0.43; 95% CI, 0.34-0.56; P<0.001). The objective response rate was 40.0% in nivolumab arm compared to 13.9% in the dacarbazine group (HR =4.06; P<0.001). The most common AEs associated with nivolumab were fatigue (19.9%), pruritus (17%), and nausea (16.5%). Only 11.7% were grade 3 or 4 AEs (15).
The synergistic effect of anti CTLA-4 and anti-PD-1 antibodies was studied in a phase I trial of concurrent or sequential administration of nivolumab and ipilimumab at different doses (54). At the maximum doses that were associated with an acceptable tolerability (nivolumab 1 mg/kg and ipilimumab 3 mg/kg), objective response rate was 53%, and all responses had a tumor reduction of 80% or greater. Grade 3 or 4 AEs occurred in 53% but were generally reversible. A sequenced regimen showed a lower objective response rate (20%) with a more tolerable toxicity profile (18% grade 3-4). Long-term analysis of this trial reported a survival rate of 79% at 2 years (16). A phase II trial confirmed these efficacy results: in the CheckMate 069 trial, 142 treatment naïve metastatic melanoma patients were enrolled in a double-blind study (17) and randomized in a 2:1 ratio to ipilimumab (3 mg/kg for 4 doses) combined with either nivolumab (1 mg/kg every 2 weeks) or placebo (Table 1). Although patients could be included regardless of BRAF status, the primary endpoint was objective response rate among patients with BRAF V600 wild-type tumors. In this population, the rate of confirmed objective response was 61% in the combination group vs. 11% in the ipilimumab group (P<0.001), with 22% of complete responses in the combination regimen. The median PFS was not reached with the combination therapy and was 4.4 months with ipilimumab monotherapy (HR =0.40; 95% CI, 0.23-0.68; P<0.001). Similar results for response rate and PFS were observed in BRAF mutation-positive melanoma patients. The combination therapy showed higher grade 3-4 toxicity than ipilimumab monotherapy (54% vs. 24%), but this was manageable with immune-modulating medication (17).
As ipilimumab was approved by the FDA in previously untreated metastatic melanoma patients on the basis of improvement in OS, this drug was selected as control arm (standard of care) in the phase III trial CheckMate 067, designed to compare nivolumab monotherapy with ipilimumab, and nivolumab-ipilimumab combination vs. ipilimumab (Tables 1,3,4). The study was not designed to provide a formal statistical comparison between nivolumab and the combination with ipilimumab. A total of 945 patients were randomized in a ratio of 1:1:1 to nivolumab monotherapy, nivolumab-ipilimumab combination and ipilimumab monotherapy. Co-primary endpoints were PFS and OS. The study included 31.5% of BRAF mutant patients not previously treated. The median PFS was 11.5 months with nivolumab plus ipilimumab, compared with 2.9 months with ipilimumab (HR =0.42; 99.5% CI, 0.31-0.57; P<0.001) and 6.9 months with nivolumab (HR =0.57; 99.5% CI, 0.43-0.76; P<0.001). The objective response rates were 43.7% in the nivolumab group [overall response (OR) =3.4; 95% CI, 2.02-5.72], 57.6% in the combination arm (OR =6.11; 95% CI, 3.59-10.38) and 19% in the ipilimumab group. Grade 3 or 4 AEs occurred in 16.3% of patients in the nivolumab group, 55.0% in the nivolumab plus ipilimumab group, and 27.3% in the ipilimumab group. The most common AEs were diarrhea (1.9% vs. 8.3% vs. 4.5%, respectively) and colitis (0.6% vs. 8.3% vs. 7.7%, respectively). Although the design of the trial does not allow comparison between nivolumab-ipilimumab group and nivolumab alone, it seems that PD-L1 negative tumors (less than 5% of tumor cells showing PD-L1 staining of any intensity on the cell surface in a section containing at least 100 tumor cells) might obtain more benefit from the combination than from nivolumab alone, given that in this subgroup the PFS was longer in the combination arm [11.2 months (95% CI, 8.0- not reached) vs. 5.3 months (95% CI, 2.8-7.1)] (18). There were no significant differences in terms of median PFS for BRAF mutant patients when treated with nivolumab (5.62 months) vs. ipilimumab (4.04 months) (HR =0.77; 95% CI, 0.54-1.09); significant differences were found in the BRAF mutant subgroup when treated with the combination of nivolumab and ipilimumab (11.7 months) vs. ipilimumab (4 months) (HR =0.47; 95% CI, 0.32-0.68). There were significant differences in terms of median PFS in the BRAF wild-type subgroup: median PFS for nivolumab treated patients was 7.9 vs. 2.8 months for those treated with ipilimumab (HR =0.5; 95% CI, 0.39-0.63): BRAF wild-type patients treated with the combination of nivolumab and ipilimumab had a median PFS of 11.24 months that was significantly higher than median PFS for ipilimumab alone (HR =0.41; 95% CI, 0.32-0.53). On basis of the results of these trials, the FDA and EMA approved nivolumab for first and subsequent lines of treatment for metastatic melanoma regardless of BRAF status. The FDA has also just approved the combination regimen of nivolumab-ipilimumab for BRAF wild-type metastatic melanoma.
At the annual congress of the European Society of Medical Oncology (ESMO) 2015, data were presented from the phase II trial CheckMate 064 which analyzed safety and efficacy of nivolumab and ipilimumab when given sequentially with a planned switch. The study randomized 70 patients to nivolumab 3 mg/kg every 2 weeks for 6 cycles, followed by 4 doses of ipilimumab 3 mg/kg every 3 weeks, continued with nivolumab at the same doses (cohort A) and another 70 patients on ipilimumab 3 mg/kg every 3 weeks for 4 doses followed by nivolumab 3 mg/kg every 2 weeks (cohort B), until unacceptable toxicity occurred. The primary endpoint was incidence of treatment-related grades 3-5 AEs during the induction periods. There were grades 3-5 AEs in 50.0% and 42.9% of patients in cohorts A and B, respectively. AEs led to discontinuation of study treatment in 36.8% of patients in cohort A, and in 31.4% of patients in cohort B. The confirmed ORR at week 25 was 41.2% (95% CI, 29.4-53.8%) in patients receiving nivolumab followed by ipilimumab induction compared to 20.0% (95% CI, 11.4-31.3) in patients receiving ipilimumab followed by nivolumab (55).
Predictive factors of activity to nivolumab in melanoma are not fully understood; PD-L1 IHC using a cut off of 5% with the Dako 28-8 antibody demonstrated higher response rate in PD-L1 positive patients (44-63%), but PD-L1 negative patients also had considerable activity (19-35%), and differences in survival are not so clear (15). When nivolumab is used in combination with ipilimumab, PD-L1 expression by IHC has no predictive value (17).
Among the anti PD-1 antibodies in development are others that have been studied in melanoma. The anti–PD-1 pidilizumab is a humanized immunoglobulin (Ig) G1 mAb. In patients with metastatic melanoma, although the response rate was low (ORR 6%), OS at 12 months was 64.5%. These results remain to be confirmed in other studies since 57% of patients included in the study were treated after progression to ipilimumab (56).
Other checkpoint inhibitors in development
Anti-PD-L1 antibodies
BMS-936559
Also known as MDX-1105, BMS-936559 is an anti-PD-L1 humanized IgG4 mAb which has been tested in a phase I/II study in pretreated patients with solid tumors, including melanoma (Figure 1). The ORR was 17%, with five long lasting responders of 52 evaluable melanoma patients (57).
Atezolizumab (MPDL3280A)
A high-affinity anti-PD-L1 human IgG1 mAb tested in a phase I study in 45 patients with advanced melanoma with an ORR of 28% and PFS at 24 weeks of 43%. Grade 3-4 AEs were uncommon (58,59). Phase Ib studies are ongoing to evaluate the combination of MPDL3280A with vemurafenib or cobimetinib in melanoma (NCT01656642). Analysis of the predictive value of PD-L1 expression by IHC on infiltrating lymphocytes has better correlation with response than PD-L1 expression on cancer cells (60).
Avelumab (MSB0010718C)
A phase I trial in patients with various solid tumors, including melanoma, is ongoing (NCT01772004). Also other phase Ib/II of avelumab in combination with PF 05082566, a novel fully humanized IgG2 mAb agonist of 4-1BB (CD137, TNFRSF9), is planned (NCT02554812).
Durvalumab (MEDI4736)
Durvalumab is a human immunoglobulin G1ĸ mAb that blocks PD-L1. Results from a phase I study including a total of 26 patients (9 melanomas) tested dose escalation from 0.1 to 10 mg/kg and 15 mg/kg every 3 weeks. No dose limiting toxicities were seen. All AEs were grade 1-2 and the most frequent were asthenia, rash, and diarrhea (12% each). One melanoma patient had a partial response (61).
A phase I study evaluated the safety and efficacy of durvalumab at 3 or 10 mg/kg IV every 2 weeks in combination with dabrafenib 150 mg twice daily and trametinib 2 mg daily, or trametinib alone. The most frequent drug-related AEs were pyrexia (63%), fatigue (54%), diarrhea (30%), rash (25%) and vomiting (67%). Two patients discontinued treatment due to drug-related AEs (reversible grade 3 thrombocytopenia and reversible grade 3 choroidal effusions). Responses were observed mainly in the arms that included dabrafenib (16 of 21 patients had an objective response in cohorts treated with dabrafenib vs. 6 of 20 patients treated only with trametinib in combination with durvalumab (62). There are several trials ongoing with durvalumab, most in combination with other drugs, such as the open-label phase Ib/II study of IMCgp100 as a single agent and in combination with durvalumab and/or tremelimumab in melanoma patients, both in first line and after progression to an anti PD-1 drug (NCT02535078). Recent biologic evidence indicates that optimal responses to PD-1 directed therapy require the presence of CD8+ T cells in the tumor microenvironment and thus therapies such as IMCgp100 that recruit these effector cells to the tumor may overcome pre-existing resistance to checkpoint blockade. This emerging biology of checkpoint inhibitor resistance suggests the combination of IMCgp100 with checkpoint inhibition may have enhanced activity in patients with pre-existing resistance (63). Other clinical studies in solid tumors are testing different combinations of durvalumab such as the anti–PD-1 MEDI0680 (NCT02118337) with the OX40 agonist MEDI6469 (NCT02205333), with the anti-chemokine (C-Cmotif) receptor 4(CCR4) antibody mogamulizumab (NCT02301130), with the indoleamine 2,3-dioxygenase(IDO1)inhibitor INCB024360 (NCT02318277) and with the anti CD-73 MEDI9447. MEDI9447 reduces the extracellular production of adenosine by CD73, reducing the immunosuppressive effects of adenosine (64) (NCT02503774). Other trials are investigation combination of durvalumab with ibrutinib—an anti BTK and ITK antibody (65,66) (NCT02403271) and with the anti CTLA-4 antibody tremelimumab (NCT02261220).
mAb targeting CD40
The cell-surface molecule CD40 is a member of the TNFR superfamily and is a critical mediator of immune activation. Ligation of CD40 on APCs mediates direct immune activation, including upregulation of costimulatory molecules and other immune mediators (Figure 1). CD40 agonistic agents therefore have been developed as potential therapy for cancer, with promising results in early studies (67).
CP-870,893, a fully human IgG2 mAb, is the most potent CD40 agonist, and in multiple phase I studies has been evaluated for safety and optimal dose and schedule (68-70). Its activity in melanoma has been described in a case report of a patient with a complete response (71) and a phase I trial in solid tumors in combination with chemotherapy (three melanoma patients presented partial response) (69). Nevertheless, its activity as single agent is poor (72) and there are no ongoing trials with this drug.
Dacetuzumab (SGN-40) is a humanized IgG1 agonist mAb that targets CD40. Its clinical development has focused on hematological malignances (73). There are currently no ongoing clinical trials in solid tumors.
mAb targeting OX40
OX40 is a TNF family member described as a costimulatory molecule that is expressed transiently at the surface of CD4+ and CD8+ T cells upon activation. It promotes T cell survival and expansion. Preclinical data demonstrated that OX-40 agonists increase antitumor immunity.
Anti-OX40 mAb (9B12) is a murine IgG1 agonist mAb targeting OX40. In a phase I trial regressions and stabilizations in 25% of patients, including melanoma, were observed (74).
MEDI6469 is an OX-40 agonist currently being tested in a phase Ib/II study in combination with anti CLA-4 tremelimumab, anti PD-L1 MEDI4736 or rituximab (NCT02205333).
mAb targeting CD137
CD137 receptor (4-1BB) and its ligand are members of the TNF family expressed on activated T cells (Figure 1).
Urelumab (BMS-663513) is a fully humanized anti-CD137 agonist mAb that has been tested in a phase I dose-escalation study. Three of 54 melanoma patients included had an objective response to the treatment (75). Preclinical data demonstrated synergistic activity with anti PD-1 antibodies (nivolumab) (76). Several clinical trials have been completed or are ongoing with urelumab as single agent (NCT00612664, NCT00309023, NCT02534506), or in combination with other drugs (NCT00803374, NCT02253992, NCT00351325).
mAb targeting GITR
GITR is up-regulated in activated T cells, and after TCR activation, increase proliferation, activation, and cytokine production of CD4+ and CD8+ T cells (Figure 1). GITR also affects Tregs and agonist antibodies against GITR, destabilizes intratumoral Tregs, allowing for more efficient cytolysis by CD8+ T cells, and has demonstrated efficacy in preclinical melanoma models (77).
TRX518 is a humanized agonist anti-human GITR Ab that is being tested in a phase I clinical trial solid tumors (NCT01239134).
Anti-LAG-3 antibodies
LAG3 is a transmembrane protein that belongs to the Ig superfamily that binds to MHC class II and acts as a negative checkpoint regulator (Figure 1). Simultaneous LAG-3 and PD-1 blockade in mice led to decrease of tumor growth by synergistically enhancing antitumor immunity (78).
IMP321 is a soluble LAG-3 Ig fusion protein and MHC class II agonist. A phase I trial in pancreatic cancer demonstrated good tolerance without any AEs (79). There have been trials in melanoma, but results have not been reported (NCT01308294) and currently there are no ongoing clinical trials.
BMS-986016 is an anti-LAG-3 antibody currently in clinical trials. An ongoing phase I trial is testing BMS-986016 alone and in combination with anti PD-1 nivolumab in melanoma patients after progression to ipilimumab and to anti PD-1/PD-L1 antibody (NCT01968109).
Killer-cell immunoglobulin-like receptor (KIR) inhibitory mAb
KIRs are a family of cell surface proteins present on NK cells which regulate killing function of NKs by interacting with MHC class I molecules (Figure 1). This interaction allows them to detect tumor cells that usually have a low level of class I MHC on their surface. Most KIRs are inhibitory, meaning their recognition of MHC suppresses the cytotoxic activity of their NK cell.
Lirilumab (BMS986015) was developed as a fully human mAb anti-KIR and is currently in phase I trials in combination with anti PD-1 nivolumab (NCT01714739). A trial in combination with ipilimumab was closed on December 2014 and results have not been communicated.
Targeting transforming growth factor-beta (TGFβ)
In advanced cancers, TGFβ suppresses host antitumor immunity and promotes tumor growth and metastases.
Fresolimumab (GC1008) is a human anti-TGFβ mAb that has been tested in a phase I trial in patients with melanoma (n=27) and renal cell carcinoma (n=1). It demonstrated acceptable safety and an objective response in a melanoma patient (80). There are no clinical trials ongoing.
Galunisertib (LY2157299) is a small molecule that inhibits the TGFβ receptor I. In the first in human trial, galunisertib was well tolerated, with activity in gliomas (81). Galunisertib is being investigated either as monotherapy or in combination with standard antitumor regimens (including nivolumab) (NCT02423343)
Other checkpoints exist that could be potential targets for drug development:
T-cell immunoglobulin domain and mucin domain-3 (TIM3)
TIM3 is an inhibitory molecule expressed on T cells, Tregs, monocytes, dendritic cells, and macrophages. Binding of Gal-9 to TIM-3 causes an inhibitory signal, resulting in apoptosis of Th1 cells and cytotoxic CD8 T cells in vitro. Similarly, TIM-3 and PD-1 are co-expressed on most CD4 and CD8 T cells infiltrating solid tumors or in hematologic malignancy in mice and these cells are dysfunctional (Figure 1). TIM-3 and PD-1 expression is also up-regulated on exhausted tumor-specific CD8 T cells in the blood of melanoma and lymphoma patients. As a result, TIM-3 blockade and Treg depletion have a synergistic effect on tumor growth inhibition. TIM-3 not only suppresses antitumor response, but also promotes development of melanoma. The combination of an antibody against TIM-3 with either anti CTLA-4 or anti PD-1 increased antitumor efficacy (82).
B and T lymphocyte attenuator (BTLA)
BTLA is structurally related to CTLA-4 and PD-1. The binding of BTLA to its ligand, herpes virus entry mediator (HVEM, expressed on melanoma cells), results in decreased T-cell proliferation and cytokine production. HVEM is capable of either T-cell activation or deactivation (Figure 1).
CD160
CD160 is a glycosylphosphatidylinositol (GPI)-anchored protein that is a ligand for HVEM with a function which is not yet well understood. CD160 could be an inhibito ry checkpoint for T cells because the interaction of CD160 with HVEM results in anergy (Figure 1). Blocking CD160 with an antibody produces decreased T-cell proliferation and cytokine production restriction (83).
V-domain Ig suppressor of T-cell activation (VISTA)
VISTA is a checkpoint inhibitor that negatively regulates T-cell responses (Figure 1). VISTA is predominantly expressed on hematopoietic cells, and in multiple murine cancer models is found at particularly high levels on tumor-infiltrating myeloid cells. Preclinical studies with VISTA blockade have shown improvements in antitumor T-cell responses, leading to inhibition of tumor growth (84-86).
A2aR antagonists
Adenosine in the immune microenvironment leads to the activation of the A2a receptor that has been shown to represent one such negative feedback loop; adenosine is found at relatively high concentrations in the tumor microenvironment. To this end, blocking A2a receptor activation has the potential to markedly enhance anti-tumor immunity in mouse models. Adenosine in tumor microenvironment inhibits antitumor cytotoxic lymphocyte responses. Although T cells express inhibitory adenosine A2A receptors (A2AR) that suppress their activation, myeloid cell A2ARs also have activity in suppressing the immune response to tumors. Myeloid cell A2ARs have direct myelosuppressive effects through suppression of T cells and NK cells in the tumor microenvironment. Tumor-associated myeloid cells, such as macrophages, DCs, and myeloid derived suppressor cells (MDSCs) all express immunosuppressive A2ARs that are potential targets of adenosine receptor blockers to enhance immune response against tumors (87,88).
Conclusions
The development of humanized and very selective mAbs, design to block CTLA-4 and PD-1/PD-L1 is changing the therapeutic research landscape in oncology.
An impressive series of successful clinical trials, initiated with the anti-CTLA-4 agent ipilimumab (19,23), and were followed with two different anti PD-1 antibodies: pembrolizumab (10,11) and nivolumab (14,15,89); as well as the combination of ipilimumab and nivolumab (18,90). These studies have established also the bases for the clinical development of this approach in other tumors.
Ipilimumab as a single agent, with a low response rate, has remarkable activity in terms of long term survivors. Prospective clinical trials in the first and second-line setting, as well as, the results obtained in a pool analysis with more than 1,800 patients treated in the real-life setting and in clinical trials, demonstrate 20% of long term survivors (6).
Pembrolizumab and nivolumab have demonstrated their superiority over ipilimumab in first line setting and also after progression to ipilimumab. Activity is clear in terms of response rate, PFS and median OS, but long term follow-up is needed in order to confirm if they will achieve long survivors as ipilimumab have demonstrated (91).
The combination of ipilimumab and nivolumab is the most active immunotherapy in metastatic melanoma, both in terms of response rate and median PFS (57.6% vs. 43.7% vs. 19%, and 11.5 vs. 6.9 vs. 2.9 months) for the combination, nivolumab and ipilimumab respectively (18,92,93).
However, despite impressive results obtained with immune checkpoint inhibitors in advanced melanoma, several points need special attention. It must be emphasized that specific immunological and clinical training regarding the use of these agents is required for its optimal management, as well as the fact that a longer follow up of the current trials is still needed to definitely clarify its role, particularly in the adjuvant setting and BRAF mutated tumors.
Further studies in the coming years with the new agents, new sequences and combinations will clarify the most active approaches in melanoma as well as its role in other tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kroemer G, Galluzzi L. Combinatorial immunotherapy with checkpoint blockers solves the problem of metastatic melanoma-An exclamation sign with a question mark. Oncoimmunology 2015;4:e1058037. [PubMed]
- Okazaki T, Okazaki IM, Wang J, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 2011;208:395-407. [PubMed]
- Fourcade J, Sun Z, Pagliano O, et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8+ T cells induced by melanoma vaccines. Cancer Res 2014;74:1045-55. [PubMed]
- Lines JL, Pantazi E, Mak J, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res 2014;74:1924-32. [PubMed]
- Hamid O. Immunotherapy for melanoma using programmed death 1 checkpoint inhibitors. Clin Adv Hematol Oncol 2014;12:782-4. [PubMed]
- Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 2015;33:1889-94. [PubMed]
- Delyon J, Maio M, Lebbé C. The ipilimumab lesson in melanoma: achieving long-term survival. Semin Oncol 2015;42:387-401. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL). J Clin Oncol 2014;32:abstr LBA9000^.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [PubMed]
- Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol 2013;31:4311-8. [PubMed]
- Hodi FS, Sznol M, Kluger HM, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. J Clin Oncol 2014;32:abstr 9002.
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [PubMed]
- Sznol M, Kluger HM, Callahan MK, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol 2014;32:abstr LBA9003^.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- McDermott D, Lebbé C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev 2014;40:1056-64. [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [PubMed]
- Tchekmedyian S, Glaspy J, Korman A, Keler T, Deo Y. T. D. MDX-010 (human anti-CTLA4): a phase I trial in malignant melanoma. Proc Am Soc Clin Oncol 2002. Abstract 56.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155-64. [PubMed]
- Hersh EM, O'Day SJ, Powderly J, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs 2011;29:489-98. [PubMed]
- Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009;15:5591-8. [PubMed]
- Weber JS, O'Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008;26:5950-6. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522-30. [PubMed]
- Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol 2015;27:3-10. [PubMed]
- Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol 2009;27:1075-81. [PubMed]
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22. [PubMed]
- Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695-710. [PubMed]
- Bulliard Y, Jolicoeur R, Windman M, et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med 2013;210:1685-93. [PubMed]
- Eroglu Z, Kim DW, Wang X, et al. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer 2015. [Epub ahead of print]. [PubMed]
- Berrocal A, Arance A, Lopez Martin JA, et al. Ipilimumab for advanced melanoma: experience from the Spanish Expanded Access Program. Melanoma Res 2014;24:577-83. [PubMed]
- Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 2010;116:1767-75. [PubMed]
- Ji RR, Chasalow SD, Wang L, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012;61:1019-31. [PubMed]
- Linnemann C, van Buuren MM, Bies L, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med 2015;21:81-5. [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [PubMed]
- FDA Accepts Supplemental Biologics License Application (sBLA) for KEYTRUDA® (pembrolizumab), Merck’s Anti-PD-1 Therapy, for First-Line Treatment of Advanced Melanoma, and Grants Priority Review. Available online: http://www.businesswire.com/news/home/20150818005715/en/FDA-Accepts-Supplemental-Biologics-License-Application-sBLA
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Daud A, Ribas A, Robert C, et al. Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. J Clin Oncol 2015;33:abstr 9005.
- Kefford R, Ribas A, Hamid O, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol2014;32:abstr 3005^.
- Daud AI, Hamid O, Ribas A, et al. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma(MEL): Correlation of tumor PD-L1 expression with outcome. [abstract]. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5-9; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2014;74:Abstract nr CT104.
- Daud A, Hamid O, Robert C, et al. Relationship between programmed death ligand 1 (PD-L1) expression and clinical outcome in patients with melanoma treated with pembrolizumab (MK-3475). Eur J Cancer 2014;50:48-9.
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [PubMed]
- Ribas A, Robert C, Hodi FS, et al. Association of response to programmed death receptor 1 (PD-1) blockade with pembrolizumab (MK-3475) with an interferon-inflammatory immune gene signature. J Clin Oncol 2015;33:abstr 3001.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [PubMed]
- Hodi FS, Gibney G, Sullivan R, et al. An open-label, randomized, phase 2 study of nivolumab (NIVO) given sequentially with ipilimumab (IPI) in patients with advanced melanoma (CheckMate 064). Ann Oncol 2015;23LBA.
- Atkins MB, Kudchadkar RR, Sznol M, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. J Clin Oncol 2014;32:abstr 9001.
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol 2013;31:abstr 9010.
- Hodi FS, Kluger HM, Sullivan RJ, et al. Clinical activity of the PD-L1 inhibitor MPDL3280A in patients with metastatic melanoma: updated phase I data Pigment Cell Melanoma Res 2014;27:1169. [abstract].
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [PubMed]
- Lutzky J, Antonia SJ, Blake-Haskins A, et al. A phase 1 study of MEDI4736, an anti–PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol 2014;32:abstr 3001^.
- Ribas A, Butler M, Lutzky J, et al. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol 2015;33:abstr 3003.
- Bossi G, Buisson S, Oates J, et al. ImmTAC-redirected tumour cell killing induces and potentiates antigen cross-presentation by dendritic cells. Cancer Immunol Immunother 2014;63:437-48. [PubMed]
- Liang D, Zuo A, Shao H, et al. Roles of the adenosine receptor and CD73 in the regulatory effect of γδ T cells. PLoS One 2014;9:e108932. [PubMed]
- Sagiv-Barfi I, Kohrt HE, Czerwinski DK, et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015;112:E966-72. [PubMed]
- Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539-49. [PubMed]
- Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol 2007;25:876-83. [PubMed]
- Nowak AK, Cook AM, McDonnell AM, et al. A phase 1b clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann Oncol 2015. [Epub ahead of print]. [PubMed]
- Vonderheide RH, Burg JM, Mick R, et al. Phase I study of the CD40 agonist antibody CP-870,893 combined with carboplatin and paclitaxel in patients with advanced solid tumors. Oncoimmunology 2013;2:e23033. [PubMed]
- Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013;19:6286-95. [PubMed]
- Bajor DL, Xu X, Torigian DA, et al. Immune activation and a 9-year ongoing complete remission following CD40 antibody therapy and metastasectomy in a patient with metastatic melanoma. Cancer Immunol Res 2014;2:1051-8. [PubMed]
- Rüter J, Antonia SJ, Burris HA, et al. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther 2010;10:983-93. [PubMed]
- de Vos S, Forero-Torres A, Ansell SM, et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J Hematol Oncol 2014;7:44. [PubMed]
- Curti BD, Kovacsovics-Bankowski M, Morris N, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013;73:7189-98. [PubMed]
- Sznol M, Hodi FS, Margolin K. Phase I study of BMS-663513 a fully human anti-CD137 agonist monoclonal antibody, in patientswithadvancedcancer. J Clin Oncol 2008;26:3007. (Meeting Abstracts).
- Sanmamed MF, Rodriguez I, Schalper KA, et al. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2-/-IL2Rγnull Immunodeficient Mice. Cancer Res 2015;75:3466-78. [PubMed]
- Cohen AD, Schaer DA, Liu C, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One 2010;5:e10436. [PubMed]
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72:917-27. [PubMed]
- Wang-Gillam A, Plambeck-Suess S, Goedegebuure P, et al. A phase I study of IMP321 and gemcitabine as the front-line therapy in patients with advanced pancreatic adenocarcinoma. Invest New Drugs 2013;31:707-13. [PubMed]
- Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 2014;9:e90353. [PubMed]
- Rodón J, Carducci M, Sepulveda-Sánchez JM, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 2015;33:357-70. [PubMed]
- Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-Targeted Antitumor Immunotherapy. Cancer Res 2011;71:6567-71. [PubMed]
- Cai G, Anumanthan A, Brown JA, et al. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 2008;9:176-85. [PubMed]
- Liu J, Yuan Y, Chen W, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A 2015;112:6682-7. [PubMed]
- Wang L, Le Mercier I, Putra J, et al. Disruption of the immune-checkpoint VISTA gene imparts a proinflammatory phenotype with predisposition to the development of autoimmunity. Proc Natl Acad Sci U S A 2014;111:14846-51. [PubMed]
- Lines JL, Sempere LF, Broughton T, et al. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2014;2:510-7. [PubMed]
- Mediavilla-Varela M, Luddy K, Noyes D, et al. Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth. Cancer Biol Ther 2013;14:860-8. [PubMed]
- Cekic C, Day YJ, Sag D, et al. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 2014;74:7250-9. [PubMed]
- Deeks ED. Nivolumab: a review of its use in patients with malignant melanoma. Drugs 2014;74:1233-9. [PubMed]
- Nivolumab + ipilimumab ups melanoma response. Cancer Discov 2015;5:OF2. [PubMed]
- Faghfuri E, Faramarzi MA, Nikfar S, et al. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev Anticancer Ther 2015;15:981-93. [PubMed]
- Valsecchi ME. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:1270. [PubMed]
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:1270-1. [PubMed]