HPV-p53-miR-34a axis in HPV-associated cancers
Introduction
MicroRNAs (miRs) are non-coding regulatory RNAs that target mRNAs by binding to complementary regions of the 5' and 3' untranslated regions to regulate mRNA expression and degradation (1-3). In humans, several hundred genes have been identified to encode up to 1,000 miRs (4,5). These miRs regulate expressions and functions of approximately one third of human genes and play important roles in different cellular processes such as cell proliferation and apoptosis. MiRs may function as oncogenes (called as oncomiRs) that are associated with carcinogenesis, malignant transformation, and metastasis. While some oncomiRs are oncogenes, in that overexpression of these oncomiRs leads to cancerous growth, the other miRs are tumor suppressors in a normal cell. But, aberrant expression of the tumor suppressor miRs due to under-expressions of the corresponding genes also leads to cancerous growth, which is one of the multiple mechanisms of carcinogenesis (1-3,6).
It has been well known for a long time that infection with human papillomaviruses (HPVs) causes many cancers including cervical cancer, head and neck cancer, skin cancer and so on (7-11). HPV infections account for about 10-20% of total cancer incidence. It is also well known now that expression of high risk HPV oncogenes (E6 and E7) can alter multiple intracellular signalling pathways (12-14). These signalling pathways play crucial roles in regulating cell proliferation and apoptosis through the altered gene expression and protein modification. Recently, the research advances in molecular pathology have demonstrated that many miRs are involved in HPV-induced carcinogenesis and metastasis (15-18). Several independent studies have identified that as the most prevalent p53-induced miRNAs the members of the tumour suppressor miR-34 family are frequently altered in many human cancers. In this short review, thus, we focus on discussing the key roles of miR-34a, one of the miR-34, in HPV-induced cancers.
E6 oncogene/p53/miR-34a axis and HPV-induced carcinogenesis
Infection with HPVs causes human cancers via the oncogenic roles of expression of E6 and E7. Expression of the two oncogenes either from genes-integrated host genomes or viral episomes activates different signalling pathways to promote cell proliferation and prevent cell apoptosis (9,12,19). p53 is a well-known tumour suppressor, whose biological effects are largely due to its cellular functions, which mediate cell growth arrest by regulating expression of different cell cycle genes through the cell cycle pathway (20,21) and induce cellular apoptosis by regulating gene expression such as bcl-2 and bax through the mitochondrial apoptotic pathway (22). N-terminally truncated p53 family isoforms (ΔNp53, ΔNp63, and DNp73) play a critical role in carcinogenesis by counteracting cell cycle arrest and apoptosis (21). High-risk HPV E6 proteins bind to p53 to inhibit and degrade this tumour suppressor to abolish its cancer prevention effects. The abolished p53 cancer prevention effects include the decrease of cell apoptosis and accumulation of gene mutations and inappropriate response to DNA damage, which co-operate with other cellular changes, eventually leading to carcinogenesis (23-25). It is clear now that both benign and oncogenic HPV E6 proteins can bind to p53 by a region of C-terminal sequence (26,27). However, an N-terminal sequence is required for degradation; only oncogenic HPV’s E6 proteins have such a conserved N-terminal sequence to degrade p53 (26,28,29), which is ubiquitin-mediated (30,31).
p53 binds and activates the promoter of the gene that encodes miR-34a to up-regulate expression of miR-34a (32-34). Processing of the primary transcript into mature miR-34a involves the excision of a 30 kb intron (34). Oncogenic HPV infection interrupts the expression of miR-34a through viral oncoprotein E6; E6 knockdown increases levels of both p53 and miR-34a (35). Thus, reduced miR-34a expression is associated with high-risk HPV infection in the p53-dependent pathway (36). Furthermore, significantly reduced expression of pri-miR-34a occurs not only in cervical cancer, but also in precancerous lesion even before morphologic change and cervical cancer-derived cell lines (35,36). The miR-34a levels detected were significantly lower in cervical intraepithelial neoplasia (CIN)2-3 than in CIN1 (37,38). Li et al. showed that miR-34a was reduced in both normal and damaged cervical tissues in HPV positive women (36). However, it has also been reported that miR-34a is increased in HPV-associated cancer (39,40). It has been reported that inactivation of miR-34a strongly attenuates p53-mediated apoptosis in cells exposed to genotoxic stress, whereas overexpression of miR-34a mildly increases apoptosis. Hence, miR-34a is a direct proapoptotic transcriptional target of p53 that can mediate some of p53’s biological effects. Perturbation of miR-34a expression, as occurs in some human cancers, may thus contribute to tumorigenesis by attenuating p53-dependent apoptosis (34). The high-risk HPV E6-caused inhibition of miR-34a expression in the p53-dependent pathway is probably an early-onset event in the development of cervical cancer. Moreover, reduced miR-34a expression by HPV proteins appears to be correlated with promoting persistent infection and cervical cancer development (40) and invasive cervical cancer (41).
MiR-34a mediates HPV E6-caused cellular events
As a downstream molecule of p53, miR-34a also targets many other proteins to mediate their functions by decreasing cell proliferation, promoting apoptosis and preventing carcinogenesis (33,42,43). MiR-34a also causes cancer cell senescence (44). Therefore, miR-34a plays very important roles in carcinogenesis induced by HPV infections (Figure 1) (45-47).
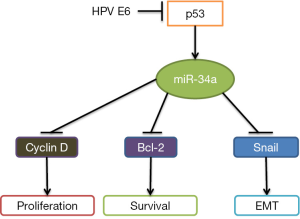
Cell proliferation and viability
Cell proliferation is a major hallmark of cancer. HPV E6 protein can increase cell proliferation through multiple signalling pathways (48-51). Decreased miR-34a due to p53 inhibition is one of the mechanisms for increased cell proliferation of HPV-associated cancers. MiR-34a has been shown to regulate cyclin E2, cyclin D1, CDK4, CDK6, E2F1, E2F3, E2F5, survivin and Sirt1 to induce cell cycle arrest that ultimately inhibits cell proliferation (42-44,52). It has also been reported that miR-34a accumulates in G0/G1 phase cells (53) and inhibits the cell cycle and proliferation of lymphoma cells by repressing its target MAP2K1 (MEK1), which is a central component of MEK/ERK signaling (54). In addition, through regulating expression of E2F3 and survivin, miR-34a overexpression could inhibit HPV-positive cancer cell viability, whereas its down-regulation promoted cell viability (55). Over-expression of miR-34a in HeLa cells and HPV negative colon cancer cell line HCT116 greatly decreased cell growth and caused moderate apoptosis (53).
Epithelial-mesenchymal transition (EMT)
EMT has been demonstrated to play a key role in HPV-associated head and neck cancer and other human cancers (56,57). Cancer cell EMT is critical for metastasis, which is regulated by transcriptional repressor Snail1. MiR-34a has been demonstrated to regulate Snail1 protein expression through binding to highly conserved 3' untranslated regions in Snail1 mRNA (58). The loss of p53 function results in decreased miR-34a levels, leading to activated Snail1-dependent EMT in colon, breast, and lung carcinoma cells. Snail was shown the only mediator for p53-caused EMT. In addition, EMT is necessary step to cause spindle-shaped mesenchyme-like phenotype (59). In cultured epithelial cell line MDCK cells, expression of HPV E6 and E7 increased mesenchymal markers including slug, twist, ZEB1and ZEB2 (60). In the cells derived from head and neck patients, ZEBs are associated with HPV16. Mendelsohn et al. showed that increased Snail in head and neck cancer is a biomarker of poor prognosis indicated by poor differentiation, increased lymphovascular invasion and metastasis (57). However, increased Snail was not associated with HPV infection in this study (57). Further study is needed to clarify the pathway of HPV E6/p53/miR-34a/Snail/EMT.
MiR-34a and P18Ink4c
P18Ink4c is a member of INK4 family, which inhibits CDK4 and CDK6. It has been shown that p18Ink4c is up-regulated in cervical cancer (53). But increased p18Ink4c did not regulate cell cycle in cervical cancer. It may be caused by E7 protein on RB or miR-34a direct regulation of CDK6 (43). MiR-34a specifically targets p18Ink4c, a CDK4 and CDK6 inhibitor induced by E2F transactivation. HPV18(+) HeLa cells with ectopic miR-34a expression or by E6 siRNA knockdown-induced expression of endogenous miR-34a exhibited a substantial reduction of p18Ink4c in a dose-dependent manner. Suppression of endogenous miR-34a in cell lines with a miR-34a inhibitor also increased p18Ink4c (53). Therefore, p18Ink4c could be a biomarker of miR-34a.
MiR-34a-Notch pathway
Notch pathway plays a critical role in carcinogenesis and metastasis of many cancers (61,62). Activation of Notch pathway also increases drug resistance to anti-cancer agents (63). Decreased Notch 1 pathway mediated by miR-34a was demonstrated to reduce cancer invasion (64). Knockdown of miR-34a increased Notch1, Jagged 1 and Hes-1 expression. In Hela and JAR cells, transfection of pre-miR-34a reduced Notch expression by 24% and 31% respectively (64). The Notch downstream target gene Hes-1 expression was also consistently reduced. Furthermore, a Notch ligand, Jagged1 that is highly expressed in colorectal cancer (CRC) has been shown to increase cancer development and metastasis (65). Knock down of Jagged1 using shRNA on CRC both in vitro and in vivo decreased colon cancer cell proliferation caused by reduced expression of cell cycle signalling molecules including Cyclin D1, Cyclin E and c-Myc. In a xenograft mouse model, inoculation of cells with knockdown of Jagged1 greatly decreased tumour growth compared with cells without knockdown (65). This was confirmed by markedly decreased cell proliferation markers (PCNA, Ki-67, and c-Myc). Knockdown of Jagged1 also decreased the migration ability of colon cancer cells (65).
MiR-34a/Bcl-2 and mitochondrial apoptotic pathway
Mitochondrial apoptotic pathway is a major pathway to cause cell apoptosis. The pathway is controlled by bcl-2 family (22,66,67). There are pro-apoptotic proteins and anti-apoptotic proteins. Bcl-2 is an anti-apoptotic protein increased in many cancers (68-70). MiR-34a has been demonstrated to target bcl-2 mRNA. Therefore, decreased levels of miR-34a in cancer can result in increased bcl-2 protein levels in several cancers (71-73). In cervical cancer, miR-34a has been shown to down-regulate bcl-2 protein expression (74). This indicates HPV-miR-34a-bcl-2 is also a mechanism for HPV-caused cancer.
Potential chemotherapeutic role of miR-34a
While several independent studies have shown that miR-34a is important in cancer prevention (45-47), other studies have suggested that miR-34a has greatly potential therapeutic role in cancer chemotherapy (75-78). Currently, chemotherapy is still a standard treatment regimen in cancer therapy. However, drug resistance is a major problem. It has been observed that miR-34a increases cancer cell sensitivity to chemotherapeutic agents (79). Therefore, miR-34a is of therapeutic importance. Decreased miR-34a was demonstrated to cause drug resistance to cisplatin in bladder cancer, to docetaxel in breast cancer and 5-FU in colon cancer (75-78).
Conclusions
MiR-34a is directly regulated by p53, which acting as a tumour suppressor is a down-streamer of the p53-network with key regulatory functions in cell proliferation, cellular apoptosis, G1-arrest, DNA repair and senescence. High-risk HPV E6 protein inhibits expression of miR-34a through the p53-pathway to increase virus-infected cell survival and enhance cancer cell proliferation and metastasis. Undoubtedly, miR-34a is a highly promising biomarker of HPV-associated cancers. Further study to characterize the expression of miR-34a in the various stages of the lesions caused by HPV infection may open a new avenue for the early diagnosis of HPV-associated cancers. Manipulation of miR-34a levels in HPV-associated cancers may have therapeutic significance.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006;6:857-66. [PubMed]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006;6:259-69. [PubMed]
- Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med 2005;353:1768-71. [PubMed]
- Landgraf P, Rusu M, Sheridan R, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007;129:1401-14. [PubMed]
- Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 2009;19:92-105. [PubMed]
- Chen J, Wang B. The roles of miRNA-143 in colon cancer and therapeutic implications. Transl Gastrointest Cancer 2012;1:169-74.
- Centers for Disease Control and Prevention (CDC). Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morb Mortal Wkly Rep 2012;61:258-61. [PubMed]
- Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health 2010;46:S20-6. [PubMed]
- Doorbar J, Egawa N, Griffin H, et al. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015;25 Suppl 1:2-23. [PubMed]
- Zhao KN, Chen J. Codon usage roles in human papillomavirus. Rev Med Virol 2011;21:397-411. [PubMed]
- McLaughlin-Drubin ME, Münger K. Oncogenic activities of human papillomaviruses. Virus Res 2009;143:195-208. [PubMed]
- Chen J. Signaling pathways in HPV-associated cancers and therapeutic implications. Rev Med Virol 2015;25 Suppl 1:24-53. [PubMed]
- Liu GB, Chen J, Wu ZH, et al. Association of human papillomavirus with Fanconi anemia promotes carcinogenesis in Fanconi anemia patients. Rev Med Virol 2015;25:345-53. [PubMed]
- McKee SJ, Bergot AS, Leggatt GR. Recent progress in vaccination against human papillomavirus-mediated cervical cancer. Rev Med Virol 2015;25 Suppl 1:54-71. [PubMed]
- Greco D, Kivi N, Qian K, et al. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS One 2011;6:e21646. [PubMed]
- Lajer CB, Garnæs E, Friis-Hansen L, et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer 2012;106:1526-34. [PubMed]
- Gardine AS, McBee WC Jr, Edwards RP, et al. MicroRNA analysis in human papillomavirus (HPV)-associated cervical neoplasia and cancer. Infect Agent Cancer 2010;5:A55.
- Wang X, Wang HK, Li Y, et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc Natl Acad Sci U S A 2014;111:4262-7. [PubMed]
- Chen J, Gu W, Yang L, et al. Nanotechnology in the management of cervical cancer. Rev Med Virol 2015;25 Suppl 1:72-83. [PubMed]
- Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature 1991;351:453-6. [PubMed]
- Engelmann D, Pützer BM. Emerging from the shade of p53 mutants: N-terminally truncated variants of the p53 family in EMT signaling and cancer progression. Sci Signal 2014;7:re9. [PubMed]
- Miyashita T, Krajewski S, Krajewska M, et al. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994;9:1799-805. [PubMed]
- Thomas M, Pim D, Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 1999;18:7690-700. [PubMed]
- Kessis TD, Slebos RJ, Nelson WG, et al. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci U S A 1993;90:3988-92. [PubMed]
- Havre PA, Yuan J, Hedrick L, et al. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995;55:4420-4. [PubMed]
- Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell 1991;67:547-56. [PubMed]
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990;248:76-9. [PubMed]
- Mansur CP, Marcus B, Dalal S, et al. The domain of p53 required for binding HPV 16 E6 is separable from the degradation domain. Oncogene 1995;10:457-65. [PubMed]
- Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol 1996;70:4509-16. [PubMed]
- Thomas M, Matlashewski G, Pim D, et al. Induction of apoptosis by p53 is independent of its oligomeric state and can be abolished by HPV-18 E6 through ubiquitin mediated degradation. Oncogene 1996;13:265-73. [PubMed]
- Hubbert NL, Sedman SA, Schiller JT. Human papillomavirus type 16 E6 increases the degradation rate of p53 in human keratinocytes. J Virol 1992;66:6237-41. [PubMed]
- He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature 2007;447:1130-4. [PubMed]
- Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 2007;26:745-52. [PubMed]
- Raver-Shapira N, Marciano E, Meiri E, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 2007;26:731-43. [PubMed]
- Wang X, Wang HK, McCoy JP, et al. Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA 2009;15:637-47. [PubMed]
- Li B, Hu Y, Ye F, et al. Reduced miR-34a expression in normal cervical tissues and cervical lesions with high-risk human papillomavirus infection. Int J Gynecol Cancer 2010;20:597-604. [PubMed]
- Gocze K, Gombos K, Kovacs K, et al. MicroRNA expressions in HPV-induced cervical dysplasia and cancer. Anticancer Res 2015;35:523-30. [PubMed]
- Tian Q, Li Y, Wang F, et al. MicroRNA detection in cervical exfoliated cells as a triage for human papillomavirus-positive women. J Natl Cancer Inst 2014.106. [PubMed]
- Gocze K, Gombos K, Juhasz K, et al. Unique microRNA expression profiles in cervical cancer. Anticancer Res 2013;33:2561-7. [PubMed]
- Ribeiro J, Sousa H. MicroRNAs as biomarkers of cervical cancer development: a literature review on miR-125b and miR-34a. Mol Biol Rep 2014;41:1525-31. [PubMed]
- Ribeiro J, Marinho-Dias J, Monteiro P, et al. miR-34a and miR-125b Expression in HPV Infection and Cervical Cancer Development. Biomed Res Int 2015;2015:304584.
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 2008;105:13421-6. [PubMed]
- Sun F, Fu H, Liu Q, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett 2008;582:1564-8. [PubMed]
- Tazawa H, Tsuchiya N, Izumiya M, et al. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A 2007;104:15472-7. [PubMed]
- Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 2012;12:613-26. [PubMed]
- Hermeking H. p53 enters the microRNA world. Cancer Cell 2007;12:414-8. [PubMed]
- Hünten S, Siemens H, Kaller M, et al. The p53/microRNA network in cancer: experimental and bioinformatics approaches. Adv Exp Med Biol 2013;774:77-101. [PubMed]
- Chen J, Elfiky A, Han M, et al. The role of Src in colon cancer and its therapeutic implications. Clin Colorectal Cancer 2014;13:5-13. [PubMed]
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther 2009;8:1313-7. [PubMed]
- Chen J, Ling MT, Shao R. Genomic instability in obesity-associated colon cancer. Transl Gastrointest Cancer 2014;3:90-97.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 2007;17:1298-307. [PubMed]
- Wang X, Meyers C, Guo M, et al. Upregulation of p18Ink4c expression by oncogenic HPV E6 via p53-miR-34a pathway. Int J Cancer 2011;129:1362-72. [PubMed]
- Ichimura A, Ruike Y, Terasawa K, et al. MicroRNA-34a inhibits cell proliferation by repressing mitogen-activated protein kinase kinase 1 during megakaryocytic differentiation of K562 cells. Mol Pharmacol 2010;77:1016-24. [PubMed]
- Geng D, Song X, Ning F, et al. MiR-34a Inhibits Viability and Invasion of Human Papillomavirus-Positive Cervical Cancer Cells by Targeting E2F3 and Regulating Survivin. Int J Gynecol Cancer 2015;25:707-13. [PubMed]
- Graves CA, Abboodi FF, Tomar S, et al. The translational significance of epithelial-mesenchymal transition in head and neck cancer. Clin Transl Med 2014;3:60. [PubMed]
- Mendelsohn AH, Lai CK, Shintaku IP, et al. Snail as a novel marker for regional metastasis in head and neck squamous cell carcinoma. Am J Otolaryngol 2012;33:6-13. [PubMed]
- Kim NH, Kim HS, Li XY, et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J Cell Biol 2011;195:417-33. [PubMed]
- Cubie HA. Experiments with human papilloma virus in cell culture. Br J Dermatol 1974;91:569-71. [PubMed]
- Jung YS, Kato I, Kim HR. A novel function of HPV16-E6/E7 in epithelial-mesenchymal transition. Biochem Biophys Res Commun 2013;435:339-44. [PubMed]
- Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther 2014;141:140-9. [PubMed]
- Shih IeM, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res 2007;67:1879-82. [PubMed]
- Martz CA, Ottina KA, Singleton KR, et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Sci Signal 2014;7:ra121. [PubMed]
- Pang RT, Leung CO, Ye TM, et al. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 2010;31:1037-44. [PubMed]
- Dai Y, Wilson G, Huang B, et al. Silencing of Jagged1 inhibits cell growth and invasion in colorectal cancer. Cell Death Dis 2014;5:e1170. [PubMed]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988;335:440-2. [PubMed]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1999;59:1693s-700s. [PubMed]
- Hockenbery DM, Oltvai ZN, Yin XM, et al. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993;75:241-51. [PubMed]
- Yang J, Liu X, Bhalla K, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997;275:1129-32. [PubMed]
- Chen J, Zhang XD, Proud C. Dissecting the signaling pathways that mediate cancer in PTEN and LKB1 double-knockout mice. Sci Signal 2015;8:pe1. [PubMed]
- Li L, Yuan L, Luo J, et al. MiR-34a inhibits proliferation and migration of breast cancer through down-regulation of Bcl-2 and SIRT1. Clin Exp Med 2013;13:109-17. [PubMed]
- Yang F, Li QJ, Gong ZB, et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol Cancer Res Treat 2014;13:77-86. [PubMed]
- Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol 2012;26:79-86. [PubMed]
- Liu L, Yu X, Guo X, et al. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol Med Rep 2012;5:753-60. [PubMed]
- Vinall RL, Ripoll AZ, Wang S, et al. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int J Cancer 2012;130:2526-38. [PubMed]
- Kastl L, Brown I, Schofield AC. miRNA-34a is associated with docetaxel resistance in human breast cancer cells. Breast Cancer Res Treat 2012;131:445-54. [PubMed]
- Li X, Khanna A, Li N, Wang E. Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;3:985-1002. [PubMed]
- Akao Y, Noguchi S, Iio A, et al. Dysregulation of microRNA-34a expression causes drug-resistance to 5-FU in human colon cancer DLD-1 cells. Cancer Lett 2011;300:197-204. [PubMed]
- Weeraratne SD, Amani V, Neiss A, et al. miR-34a confers chemosensitivity through modulation of MAGE-A and p53 in medulloblastoma. Neuro Oncol 2011;13:165-75. [PubMed]


