The critical roles of miR-21 in anti-cancer effects of curcumin
Introduction
Many risk factors have been shown to induce cancer including genetic defects, epigenetic alterations, diet, obesity and infection (1,2). These factors alter intracellular signalling pathways to promote cell proliferation and decrease apoptosis; thus increase cancer incidence (3). Activation of survival signalling pathways such as phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) and mitogen activated protein kinase (MAPK), is also associated with the cancer cell resistance to anti-cancer therapeutic agents (4-8). Therefore, manipulation of signalling pathways has been targeted for cancer treatment. Indeed, many small molecule inhibitors of signalling molecules have been developed by various companies and tested extensively (9,10). Some of them have been used in multiple clinical trials. Interestingly some phytochmicals have been revealed to have property to inhibit multiple signalling pathways in cancer cells and thus are attractive candidates for cancer prevention and treatment (11). One among them is curcumin, which has been recently studied extensively (12-16).
Curcumin is extracted from a spice called turmeric, derived from a Zingiberaceae family plant Curcuma longa L (17). Indeed, turmeric has had medicinal applications in various diseases for thousands of years in Asian countries (17-20). Curcumin can decrease cell proliferation and increase apoptosis in many cancer cell lines in vitro including colon cancer, breast cancer, prostate, lung cancer, and so on (21-23). It has been demonstrated that curcumin is effective on many cancers caused by various risk factors such as obesity-associated cancers and HPV-caused cancers. Obesity is known to increase cancer incidence and lead to vicious prognosis through activation of multiple signalling pathways (24-26). Curcumin is effective in the prevention and treatment of obesity-associated cancers in animal models (27). It has been shown to reduce colon poly formation in several animal models for obesity-associated colon cancer (28-30). Curcumin is also effective against HPV-caused cancers (31,32).
The mechanisms for the anti-cancer effects of curcumin have been suggested to be its inhibition of multiple signalling pathways and anti-oxidant property. Curcumin can inhibit several common cancer promoting pathways including MAPK, Akt, NF-κB and Cox-2 (33-35). Recent studies have shown that curcumin regulates many microRNAs (miRs) (36-38). In this review, we summarise the roles of miRNA-21 in the anti-cancer effects of curcumin and the associated mechanisms.
microRNA-21 (miR-21)
MiRs are endogenous RNAs which have 19-25 nucleotides (39-41). MiRs do not translate into proteins but can regulate gene expression. When miRs bind to complementary mRNAs, miRs can cause degradation of mRNAs or stop the translation of mRNAs and thus decrease target protein production. MiRs are involved in many physiological processes including cell proliferation, differentiation and survival (42,43). MiRs regulate about one third of gene expression in human body. It is thus not surprising that miRs play important roles in cancer development and progression. MiRs may target tumour suppressors to promote cancer formation (oncomirs) or target oncogenes to prevent cancer development (tumour suppressor miRs) (44).
MiR-21 is one of the most frequently up-regulated miRs in many cancers including breast, gastric, colon, lung, pancreatic and ovarian cancers (45,46). It can increase cell proliferation and decrease apoptosis and therefore increase cancer incidence (47). High level of miR-21 has been shown to be associated with poor prognosis in many cancer cases (48). MiR-21 stimulates multiple survival signalling pathways to mediate its roles in cancers. Therefore, targeting miR-21 has therapeutic implications.
The roles of miR-21 in various aspects of curcumin’s anti-cancer effects
Curcumin has been found to decrease miR-21, which is a key mechanism for curcumin to have anti-cancer effects (49). MiR-21 mediates several important aspects of anti-cancer effects of curcumin including cell proliferation, metastasis, stemness and sensitivity to anti-cancer therapeutic agents.
Proliferation and apoptosis
Increased cell proliferation and decreased cell apoptosis are two major cellular characteristics of cancers (50,51). Inhibiting proliferation and inducing apoptosis are thus important approaches for the prevention and treatment of cancer. Cell proliferation is determined by cell cycle which includes four phases and is regulated by cell cyclins (3). Mudduluru et al. showed that curcumin reduced colon cancer cell cycles through down-regulation of miR-21 (49). Zhang et al. showed that curcumin decreased human non-small cell lung cancer A549 cell proliferation and increased apoptosis with decreased miR-21 (52). Over-expression of miR-21 in these cells decreased the effect of curcumin on A549 cells. Therefore, the level of miR-21 is critical for curcumin to inhibit cancer cell proliferation and induce cancer cell apoptosis
Metastasis
Metastasis is the major reason for cancer to cause deaths, which undergoes several stages including cell detachment, migration, attachment and growth (53,54). Curcumin has been revealed to reduce cancer cell migration via inhibiting miR-21 in various cancers including colon cancer (49). Bao et al. showed that curcumin analogue difluorinated-curcumin (CDF) reduced pancreatic cancer cell migration via decreasing miR-21 (55). Curcumin can also cause anoikis, which refers to cell death after detachment (56,57). Whether it is mediated by miR-21 is unknown.
Cancer stem cells (CSCs)
CSCs refer to those cells that can reproduce themselves and sustain cancer (58-60). They are responsible for drug resistance in cancer treatment. Therefore, it is critical to reduce stemness of cancer cells to increase treatment efficacy. Bao et al. showed that CDF reduced pancreatosphere formation via decreasing miR-21 (55). Hypoxia increased CSC biomarkers such as Nanog, Oct4 and EZH2 mRNA expression while CDF decreased the levels of these markers (55).
Drug resistance
Drug resistance, either primary or acquired, is a major problem in the treatment of cancers (61-63). Application of curcumin has been shown to overcome drug resistance in several studies. It has been revealed that inhibition of miR-21 by curcumin could be an important mechanism. Inhibition of miR-21 by curcumin increased pancreatic cancer cell sensitivity to gemcitabine (64). Roy et al. showed that CDF decreased miR-21 in 5-FU and oxaliplatin resistant colon cancer cell lines through upregulation of phosphatase and tensin homolog (PTEN) and thus reduction of activity status of PI3K/Akt pathway (65). Activation of the PI3K/Akt pathway is well-known to cause drug resistance in colon cancer cells (5,6).
Multiple signalling pathways involved in miR-21 mediated curcumin’s anti-cancer effect
Many studies have revealed that miR-21 mediates the anti-cancer effects of curcumin via multiple signalling pathways as described below. The common pathways affected include PTEN/PI3K/Akt, NF-κB and programmed cell death protein 4 (PDCD4) (Figure 1).
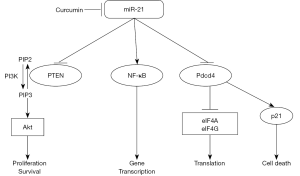
PTEN/PI3K/Akt pathway
PI3K/Akt is a common survival pathway. PI3K catalyses phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), which recruits Akt and phosphoinositide dependent kinase (PDK) to allow PDK to phosphate and activate Akt (66,67). Activated Akt increases cell proliferation and decreases cell apoptosis via a broad range of downstream target proteins. PTEN is a negative regulate of PI3K/Akt pathway by turning PIP3 into PIP2. Curcumin restored PTEN expression via inhibiting miR-21 in colon cancer (68). Induction of miR-21 suppressed the anti-cancer of curcumin through decreasing PTEN (52). MiR-21 is known to cause drug resistance to cisplatin via decreasing PTEN (69). Studies also showed that miR-21 targeted PTEN by complementing its sequence (70,71).
Programmed cell death protein 4 (PDCD4)
PDCD4 is a tumour suppressor. MiR-21 was found to be inversely related with PDCD4 in both colon cancer cell lines and tissue samples from colon cancer patients (72-74). MiR-21 complements the 3-UTR of PDCD4 and thus inhibits the translation of PDCD4 (72). Mutation in the PDCD4 region that complementary with miR-21 abolished the regulation of PDCD4 by miR-21. The PDCD4 regulated by miR-21 has been associated with cancer transformation and metastasis (72,75). MiR-21 mediated PDCD4 degradation has also been demonstrated in breast cancer (76), cervical cancer (77), Glioblastoma (78,79) and hepatocellular carcinoma (80). MiR-21 mediated PDCD4 degradation has been shown to be important in curcumin-reduced metastasis (49).
NF-κB
NF-κB is a transcriptional factor that can promote cancer development through upregulation of many oncogenes. Curcumin has been shown to decrease NF-κB via miR-21 (81). Curcumin is well known to cause decreased accumulation of NF-κB in the nuclei in many cancer cells. Yang et al. demonstrated that EF24, a curcumin analogue, caused apoptosis of prostate cancer and B16 murine melanoma cells through inhibition of NF-κB (81). The decreased miR-21 levels by EF24 may be important for NF-κB reduction. This is consistent to that miR-21 can increase NF-κB in other studies (82,83). However, it is not clear how miR-21 regulates NF-κB.
The mechanism for curcumin to decease miR-21
It has recently been found that curcumin decreases miR-21 by increasing its secretion carried in exosomes in chronic myelogenous leukemia (CML) cell lines K562 and LAMA84 (84). Exosomes are cell-derived nanosize vesicles that contain miRs and present in all biological fluids. In in vivo experiments, curcumin treatment of CML xenograft SCID mice has increased plasma exosomes containing miR-21 (84). This was accompanied by an increase in PTEN and a decrease in AKT phosphorylation in CML cells, indicating decreased miR-21 in the cells.
Curcumin has also been found to inhibit miR-21 gene promoter activity directly. A motif in miR-21 to bind to activator protein 1 (AP-1) transcription factor has been identified (49). Curcumin can inhibit AP-1 binding and thus decrease the transcription of miR-21 gene.
Conclusions
Curcumin has anti-cancer effects on many cancer cells. It can inhibit multiple signalling pathways, altering many cellular physiological processes. Recent findings showed that curcumin also affects many miRs, which are important regulators of oncogenes and tumour suppressors. Curcumin can decrease miR-21, a key oncomir, through increasing its exosome exclusion and inhibiting the transcription of miR-21 gene. Inhibition of miR-21 mediates many aspects of anti-cancer effects of curcumin including cell proliferation, apoptosis, migration, stemness and drug resistance through PTEN/PI3K/Akt, PDCD4 and NF-κB.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- McKee SJ, Bergot AS, Leggatt GR. Recent progress in vaccination against human papillomavirus-mediated cervical cancer. Rev Med Virol 2015;25 Suppl 1:54-71. [PubMed]
- Doorbar J, Egawa N, Griffin H, et al. Human papillomavirus molecular biology and disease association. Rev Med Virol 2015;25 Suppl 1:2-23. [PubMed]
- Chen J. Signaling pathways in HPV-associated cancers and therapeutic implications. Rev Med Virol 2015;25 Suppl 1:24-53. [PubMed]
- McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006;46:249-79. [PubMed]
- Chen J, Huang XF, Qiao L, et al. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol 2011;2:27-33. [PubMed]
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119-25. [PubMed]
- Martz CA, Ottina KA, Singleton KR, et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Sci Signal 2014;7:ra121. [PubMed]
- Tao JJ, Castel P, Radosevic-Robin N, et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Sci Signal 2014;7:ra29. [PubMed]
- Sawyers C. Targeted cancer therapy. Nature 2004;432:294-7. [PubMed]
- Chen J. Targeted therapy of obesity-associated colon cancer. Transl Gastrointest Cancer 2012;1:44-57.
- Chen J. Editorial: phytochemicals, intracellular signalling pathways and anti-cancer effects. Anticancer Agents Med Chem 2014;14:777-8. [PubMed]
- Park W, Amin AR, Chen ZG, et al. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387-400. [PubMed]
- Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets 2005;5:117-29. [PubMed]
- Duvoix A, Blasius R, Delhalle S, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett 2005;223:181-90. [PubMed]
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 2009;30:85-94. [PubMed]
- Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors 2013;39:56-68. [PubMed]
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 2009;41:40-59. [PubMed]
- Maheshwari RK, Singh AK, Gaddipati J, et al. Multiple biological activities of curcumin: a short review. Life Sci 2006;78:2081-7. [PubMed]
- Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr 2010;103:1545-57. [PubMed]
- Chen J. Prevention of obesity-associated colon cancer by (-)-epigallocatechin-3 gallate and curcumin. Transl Gastrointest Cancer 2012;1:243-249.
- Ravindran J, Prasad S, Aggarwal BB. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J 2009;11:495-510. [PubMed]
- Jiang MC, Yang-Yen HF, Yen JJ, et al. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr Cancer 1996;26:111-20. [PubMed]
- Choudhuri T, Pal S, Agwarwal ML, et al. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett 2002;512:334-40. [PubMed]
- Chen J, Chi M, Chen C, et al. Obesity and melanoma: exploring molecular links. J Cell Biochem 2013;114:1955-61. [PubMed]
- Chen J, Ling MT, Shao R. Genomic instability in obesity-associated colon cancer. Transl Gastrointest Cancer 2014;3:90-7.
- Chen J, Yang J, Zhao KN. FTO gene, obesity and colon cancer: from epidemiological evidence to laboratory studies. Transl Gastrointest Cancer 2013;2:194-203.
- Shehzad A, Khan S, Sup Lee Y. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol 2012;8:179-90. [PubMed]
- Kubota M, Shimizu M, Sakai H, et al. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer 2012;64:72-9. [PubMed]
- Lund EK, Belshaw NJ, Elliott GO, et al. Recent advances in understanding the role of diet and obesity in the development of colorectal cancer. Proc Nutr Soc 2011;70:194-204. [PubMed]
- Chen J, Huang XF. High fat diet-induced obesity increases the formation of colon polyps induced by azoxymethane in mice. Ann Transl Med 2015;3:79. [PubMed]
- Maher DM, Bell MC, O'Donnell EA, et al. Curcumin suppresses human papillomavirus oncoproteins, restores p53, Rb, and PTPN13 proteins and inhibits benzo[a]pyrene-induced upregulation of HPV E7. Mol Carcinog 2011;50:47-57. [PubMed]
- Prusty BK, Das BC. Constitutive activation of transcription factor AP-1 in cervical cancer and suppression of human papillomavirus (HPV) transcription and AP-1 activity in HeLa cells by curcumin. Int J Cancer 2005;113:951-60. [PubMed]
- Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. Biofactors 2013;39:27-36. [PubMed]
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 2001;1:11-21. [PubMed]
- Delage B, Rullier A, Capdepont M, et al. The effect of body weight on altered expression of nuclear receptors and cyclooxygenase-2 in human colorectal cancers. Nutr J 2007;6:20. [PubMed]
- Yang J, Cao Y, Sun J, et al. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med Oncol 2010;27:1114-8. [PubMed]
- Dahmke IN, Backes C, Rudzitis-Auth J, et al. Curcumin intake affects miRNA signature in murine melanoma with mmu-miR-205-5p most significantly altered. PLoS One 2013;8:e81122. [PubMed]
- Zhang J, Du Y, Wu C, et al. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep 2010;24:1217-23. [PubMed]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [PubMed]
- Ambros V. The functions of animal microRNAs. Nature 2004;431:350-5. [PubMed]
- Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 2006;94:776-80. [PubMed]
- Bueno MJ, Pérez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle 2008;7:3143-8. [PubMed]
- Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer 2006;6:259-69. [PubMed]
- Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene 2007;26:2799-803. [PubMed]
- Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med 2009;13:39-53. [PubMed]
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 2005;65:6029-33. [PubMed]
- Wang P, Zhuang L, Zhang J, et al. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol Oncol 2013;7:334-45. [PubMed]
- Mudduluru G, George-William JN, Muppala S, et al. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep 2011;31:185-97. [PubMed]
- He K, Qi Q, Chan CB, et al. Blockade of glioma proliferation through allosteric inhibition of JAK2. Sci Signal 2013;6:ra55. [PubMed]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411:342-8. [PubMed]
- Zhang W, Bai W, Zhang W. MiR-21 suppresses the anticancer activities of curcumin by targeting PTEN gene in human non-small cell lung cancer A549 cells. Clin Transl Oncol 2014;16:708-13. [PubMed]
- Chen J. Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat Clin Pract Gastroenterol Hepatol 2008;5:306-7. [PubMed]
- Chen J, Elfiky A, Han M, et al. The role of Src in colon cancer and its therapeutic implications. Clin Colorectal Cancer 2014;13:5-13. [PubMed]
- Bao B, Ali S, Ahmad A, et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One 2012;7:e50165. [PubMed]
- Pongrakhananon V, Nimmannit U, Luanpitpong S, et al. Curcumin sensitizes non-small cell lung cancer cell anoikis through reactive oxygen species-mediated Bcl-2 downregulation. Apoptosis 2010;15:574-85. [PubMed]
- Chanvorachote P, Chunhacha P, Pongrakhananon V. Anoikis: a potential target to prevent lung cancer metastasis? Lung Cancer Management 2013;2:169-71.
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006;355:1253-61. [PubMed]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68. [PubMed]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med 2007;58:267-84. [PubMed]
- Ozben T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett 2006;580:2903-9. [PubMed]
- Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol 2012;83:1104-11. [PubMed]
- Bock C, Lengauer T. Managing drug resistance in cancer: lessons from HIV therapy. Nat Rev Cancer 2012;12:494-501. [PubMed]
- Ali S, Ahmad A, Banerjee S, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 2010;70:3606-17. [PubMed]
- Roy S, Yu Y, Padhye SB, et al. Difluorinated-curcumin (CDF) restores PTEN expression in colon cancer cells by down-regulating miR-21. PLoS One 2013;8:e68543. [PubMed]
- Chen J, Zhang XD, Proud C. Dissecting the signaling pathways that mediate cancer in PTEN and LKB1 double-knockout mice. Sci Signal 2015;8:pe1. [PubMed]
- Hennessy BT, Smith DL, Ram PT, et al. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 2005;4:988-1004. [PubMed]
- Bao B, Ali S, Kong D, et al. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One 2011;6:e17850. [PubMed]
- Yang SM, Huang C, Li XF, et al. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology 2013;306:162-8. [PubMed]
- Qin X, Yan L, Zhao X, et al. microRNA-21 overexpression contributes to cell proliferation by targeting PTEN in endometrioid endometrial cancer. Oncol Lett 2012;4:1290-6. [PubMed]
- Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans 2009;37:918-25. [PubMed]
- Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008;27:2128-36. [PubMed]
- Chang KH, Miller N, Kheirelseid EA, et al. MicroRNA-21 and PDCD4 expression in colorectal cancer. Eur J Surg Oncol 2011;37:597-603. [PubMed]
- Fassan M, Pizzi M, Giacomelli L, et al. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch 2011;458:413-9. [PubMed]
- Talotta F, Cimmino A, Matarazzo MR, et al. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene 2009;28:73-84. [PubMed]
- Frankel LB, Christoffersen NR, Jacobsen A, et al. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 2008;283:1026-33. [PubMed]
- Yao Q, Xu H, Zhang QQ, et al. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun 2009;388:539-42. [PubMed]
- Gaur AB, Holbeck SL, Colburn NH, et al. Downregulation of Pdcd4 by mir-21 facilitates glioblastoma proliferation in vivo. Neuro Oncol 2011;13:580-90. [PubMed]
- Chen Y, Liu W, Chao T, et al. MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer Lett 2008;272:197-205. [PubMed]
- Zhu Q, Wang Z, Hu Y, et al. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol Rep 2012;27:1660-8. [PubMed]
- Yang CH, Yue J, Sims M, et al. The curcumin analog EF24 targets NF-κB and miRNA-21, and has potent anticancer activity in vitro and in vivo. PLoS One 2013;8:e71130. [PubMed]
- Li H, Jia Z, Li A, et al. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol Cell Biochem 2013;382:137-43. [PubMed]
- Sha M, Ye J, Zhang LX, et al. Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway. Pharmacology 2014;93:39-46. [PubMed]
- Taverna S, Giallombardo M, Pucci M, et al. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: a possible role for exosomal disposal of miR-21. Oncotarget 2015;6:21918-33. [PubMed]


