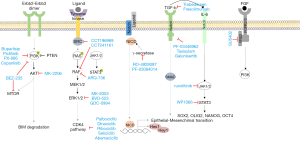
Other targeted drugs in melanoma
Introduction
Melanoma is one of the most aggressive tumors; until very recently, there were no active treatments for this disease and median survival time was less than 1 year (1).
Advances in translational research have led to the development of very active drugs for several tumor types, such as EGFR inhibitors for EGFR-mutated lung cancer (2) or imatinib for GIST tumors harboring KIT or PDGFR mutations (3). Recently the identification of the BRAF mutation in 50% of melanomas has led to development of the first targeted drugs for this disease (4). The combination of BRAF and MEK inhibitors has demonstrated an even higher activity in BRAF mutant melanomas with response rates close to 80% and a significant improvement in median survival (close to 2 years) (5-7). Nevertheless, new therapies are required due to the ability of melanomas to quickly develop resistance to current therapies. Understanding of the signaling pathways and the interactions between them could therefore provide new targets for drug development in melanoma.
Herein we review the main signaling pathways in melanoma and some of the advances in new targets and drug development for this disease (Table 1).

Full table
PAN-RAF selective inhibitors
Activation of the RAS/RAF/MEK/ERK pathway occurs in NRAS and BRAF mutant melanomas. These tumors, especially those with BRAF mutation, are dependent on ERK signal for proliferation and survival (8). The most frequent mechanisms of resistance to BRAF inhibitors are the acquisition of a new NRAS mutation, overexpression of COT, or new splicing forms of BRAF (9-11). BRAF inhibitors such as vemurafenib or dabrafenib induce this paradoxical RAF activation via RAF dimerization in BRAF wild type cells (9). This process is known as “drug-mediated RAF dimers transactivation” (12). Therefore, BRAF inhibitors are could be potentially deleterious in NRAS mutant melanomas or melanomas with NF-1 loss.
Several pan-RAF inhibitors have been developed. These new drugs inhibit all or most of the members of the RAF family, including rare variants of BRAF, splicing variants, BRAF fusion proteins, NRAS mutants (Figure 1). They, therefore, block this phenomenon and could theoretically be effective in NRAS mutant melanomas or in many BRAF mutant melanomas resistant to BRAF inhibitors (13).
TAK-632 is active for NRAS and resistant BRAF mutant melanoma cell lines either due to the development of splicing forms or to an acquired NRAS mutation (13,14). The combination of this drug with the MEK inhibitor TAK-733 has a synergistic effect on cell lines. However, there are currently no ongoing clinical trials with this drug.
ARQ-736 is another panRAF inhibitor currently in development. A phase I clinical trial for BRAF resistant melanomas or NRAS mutant melanomas has now been completed (NCT01225536) and results are awaited.
CCT196969, CCT241161: these panRAF inhibitors also inhibit RTK/SRC-family kinase (SFK) signaling. These compounds do not drive paradoxical pathway activation and inhibit MEK/ERK in BRAF and NRAS mutant melanoma. They do inhibit melanoma cells and patient-derived xenografts resistant to BRAF and BRAF/MEK inhibitors (15).
Other panRAF inhibitors in development: LY3009120, CCT3833.
ERK1/2 inhibitors
When acquired resistance to BRAF inhibitors occurs, MAPK pathway is reactivated in 80% of cases through different mechanisms. Since they all converge downstream activating ERK, ERK inhibitors may play a role in these situations (Figure 1).
Unlike BRAF or MEK inhibitors, most ERK inhibitors are still in preclinical phases of development. These inhibitors generally show activity as single agents in preclinical studies, especially in BRAF resistant mutant melanoma (16). The combination of these drugs with BRAF inhibitors is particularly interesting since they have an opposite effect on BRAF mutant cells and it is expected that the combination is less toxic than monotherapy with BRAF inhibitors or ERK inhibitors alone. The potential advantage of the combination of BRAF inhibitors and ERK inhibitors is based on the fact that ERK inhibitors block the phosphorylation of ERK, reducing the chances of developing feedback. But the question is if this combination will be superior to the standard combination of BRAF and MEK inhibitors. Some of the intrinsic differences among ERK inhibitors are related to their biochemical activities with kinetic differences in phosphorylation and dephosphorylation of ERK that suggest a range of conformational effects. Also in vitro profiles of ERK inhibitors can differ with effects on phospho-ERK levels different ERK between different inhibitors: most chemical series show rapid and sustained increase in phospho-ERK, but for example, GDC-0994 has little acute effect on phospho-ERK in vitro, in contrast to SCH772984 that is reported to decrease phospho-ERK (17). It is well-known that inhibition of phospho-p90RSK correlates well with biochemical potency, and could be a subrogated marker of activity.
It is expected that dose limiting toxicities (DLT) due to inhibition of ERK do not allow a narrow therapeutic index. The combination with BRAF inhibitors in intermittent schemes could minimize side effects and delay the onset of resistance (18).
SCH772984 is a new selective inhibitor of ERK1/2. In addition to inhibiting ERK kinase activity directly, also it inhibits ERK phosphorylation mediated by MEK, unless it is not a direct inhibitor of MEK (19). In preclinical studies, SCH772984 has demonstrated a response rate of 50% in NRAS mutant melanomas cell lines and especially in BRAF resistant melanoma through acquisition of new mutations in RAS or MEK1, splicing variants of BRAF or BRAF amplification (17) .
VTX11e is an inhibitor of ERK. In laboratory models, sensitivity is recovered when VTX11e is added to the combination of MEK inhibitors and BRAF inhibitors in resistant melanomas with NF1 loss (20).
MK-8353 is an ERK1/2 inhibitor that has completed a phase I clinical trial in multiple solid tumors (NCT01358331).
BVD-523 is an ERK1/2 inhibitor currently in a phase I study in solid tumors including untreated BRAF mutant melanomas, BRAF mutant melanomas with resistance to BRAF inhibitors and NRAS mutant melanomas (NCT01781429).
AEZS-131 is an ERK inhibitor with demonstrated activity in colorectal and breast cancer models. However, there are no clinical trials currently ongoing.
FR-180204 is an ERK1/2 inhibitor (21). There are no clinical studies currently ongoing.
GDC-0994 is a highly selective inhibitor of ERK1/2 (22). Two phase I trials are ongoing in solid tumors, one as a single agent (NCT 01875705) and the other one in combination with cobimetinib (a MEK inhibitor) (NCT02457793).
CDK4/6 inhibitors
The p16-cyclin D-CDK4/6-retinoblastoma protein (RB) pathway (CDK4 pathway) is alterated in 90% of melanomas. When CDK4 is activated, it leads to phosphorylation and, therefore, to inhibition of the RB that permits G1-S cell-cycle transition (23).
Cyclin synthesis and consequently the activation of cyclin-dependent kinase (CDK) is specific of the stages of the cell cycle, and coordinates DNA replication and cell division. CDK activity is regulated at many levels through different mechanisms including the number of regulatory cyclin subunits, its association with the catalytic unit of CDK, and the members of the two families of CDK inhibitory proteins. This family includes INK4A (p16), INK4B (p15 and CDKN2B), INK4C (p18 and CDKN2C) and INK4D (p19 or CDKN2D) and CIP, as well as the KIP family which includes p21 (CDKN1A), p27 (CDKN1B) and p57 (CDKN1C) (23).
CDKs are often overactive in cancer due to genetic and epigenetic alterations that affect either the CDKs, the CDKs activators, the endogenous inhibitors or the upstream mitogenic pathways. Although CDKs are not usually mutated in tumors, they are normally amplified or overexpressed, as are their respective cyclin activators, while their inhibitors are usually lost. In summary, the cascade of cyclin D-CDK4/6, INK4A (p16), RB and transcription factor E2F are altered in most tumors.
The relationship between the risk of melanoma and the alteration of the CDK pathway was first described when germline mutations in the CDKN2A (p16INK4A) and CDK4 were discovered (24). Currently, it is well known that loss of p16INK4A occurs in approximately 80-90% of metastatic melanomas, commonly through deletion (50%), mutation (10%) or promoter methylation (20-30%). The loss of p16 from benign nevi to metastatic melanoma is a progressive phenomenon: p16 is positive in 80% of nevi, in 30-80% of primary melanomas and in 10-20% of metastatic melanomas. This loss at different stages of disease reflects the important role of the CDK pathway in melanoma progression (23).
From the CDK family, CDK2 plays a fundamental role in the development and progression of melanoma and its expression in melanoma is regulated by the transcription factor MITF (25). Melanoma cell lines with low expression of CDK2 have increased sensitivity to CDK inhibitors (25).
CDK inhibitors are small molecule inhibitors of cyclin kinase which have been tested in several tumors as single or combination agents. In breast cancer, the combination with hormone therapy has recently achieved FDA approval (26,27).
Combination therapies with other target drugs in melanoma are supported by solid preclinical studies, both in vitro and in vivo. Trametinib, a MEK inhibitor, in combination with the CDK4/6 inhibitor palbociclib demonstrated high activity in NRAS mutant models, with 30% of complete responses in animal models (28). The first compound that inhibited CDKs was flavopiridol (Alvocidib) from Sanofi-Aventis. Unfortunately, clinical studies showed poor activity in melanoma and other tumors, probably because flavopiridol is not a specific inhibitor of CDK4 (29). Other inhibitors of CDK4 have recently entered clinical development.
Palbociclib (PD-0332991) is an inhibitor of CDK4 and CDK6 which has recently been approved for treatment of hormone receptor positive breast cancer on the basis of the survival benefit demonstrated in a phase III trial in advanced disease (27). The DLT is hematological. In melanoma, palbociclib is being tested in several phase I trials as single agent in tumors with mutant or amplified CDK4 (NCT01037790), in solid tumors in combination with the MEK inhibitor trametinib (NCT02065063) and in BRAF mutant melanoma with CDNKN2A loss in combination with the BRAF inhibitor vemurafenib (NCT02202200).
Dinaciclib (MK-7965) (MSD) is a non-selective inhibitor of CDK4/6 which also inhibits CDK2, CDK9, CDK5 and CDK1 and has a better toxicity profile than flavopiridol. Treatment of human melanoma mouse xenografts with dinaciclib led to tumor regression associated with reduced RB phosphorylation and Bcl-2 expression. It is believed that the pro-apoptotic activity is mediated by inhibition of CDK7 and CDK9 that regulates expression of the antiapoptotic proteins Bcl-2 and Mcl-1. Further mechanistic studies revealed that dinaciclib induces p53 expression whilst simultaneously down-regulating expression of the anti-apoptotic factors Mcl-1 and XIAP (30). It has been demonstrated to have single agent activity in relapsed myeloma in a phase II trial (31). In advanced melanoma, a phase II clinical trial has concluded and results are pending (NCT00937937).
Ribociclib (LEE011) (Novartis) has been evaluated in melanoma in several phase I trials in combination with BRAF or MEK inhibitors (NCT01777776, NCT01781572, NCT01820364). Early data from a phase Ib/II study has shown that the combination with the MEK1/2 inhibitor binimetinib (MEK162) is active in patients with NRAS-mutant melanoma, with objective response in seven patients from 21 evaluable (32). Seliciclib (roscovitine, CYC202) has shown clinical benefit as a single agent in phase I and II clinical studies in nasopharyngeal cancer and non-small cell lung cancer (NSCLC). Interim phase II data showed that 7 of 10 previously-treated NPC patients had stable disease with 2 staying on treatment for over 8 and 1 for over 24 months (33). In a phase I study shown a significant transcriptional down-regulation of genes related to cellular proliferation and survival in some patients post treatment. Topline results from a double-blinded, randomized phase IIb study in patients with at least two prior treatments for NSCLC showed no difference in progression free survival but an increase in median overall survival favoring seliciclib over placebo (http://investor.cyclacel.com/releasedetail.cfm?ReleaseID=614395).
Other specific inhibitors of CDK4/6 in development are LY2835210 and P276-00. These inhibitors are less toxic, but still show hematological toxicity.
Abemaciclib (LY2835210) (Lilly) is one of the most promising CDK inhibitors. Results from a phase I trial including 132 patients with five tumor types, were presented during the 2014 Association for Cancer Research meeting, showing anti-tumor activity for LY2835210 in HR+ metastatic breast cancer patients, with a response rate of 25% (9 out of 36 patients) (34). Data from a phase I study suggested a higher activity in KRAS mutant tumors, with a demonstrated a response rate of 55% in 29 KRAS mutant patients vs. 38% in those that were wild-type KRAS (35). It is being tested in several phase III clinical trials in breast and lung cancer, but there are not ongoing studies in melanoma.
P276-00 has been tested in a phase II clinical trial in melanoma with expression of cyclin D1 protein (NCT00835419).
Inactivation of RB appears to predict resistance to CDK4 and CDK6 inhibitors. Other promising biomarkers, such as loss of p16INK4a and gains of cyclin D1, failed to predict a benefit with palbociclib in the phase III trial in estrogen-receptor-positive breast cancer (27).
PI3K/AKT/mTOR inhibitors
PI3K activation initiates a signal transduction cascade, of which the major effectors are the kinases AKT and mTORC1. PTEN is a tumor-suppressor gene that functions as a phosphatase and is the primary negative regulator of PI3K through hydrolysis of PIP3 (36).
Deregulation of the PI3K pathway has frequently been implicated in a wide range of malignancies, including melanoma. Alteration of the pathway commonly occurs through mutation or amplification of PIK3CA that encodes the p110α catalytic subunit, loss of function of PTEN (mutation, deletion, or lower expression), alterations in the INPP4B and PHLPP phosphatases, mutations of the PI3K regulatory subunits, through activation of upstream receptor tyrosine kinases or cross-talk with the RAS pathway.
In the case of BRAF mutant melanoma, PI3K reactivation is involved in 20% of patients with resistance to BRAF and MEK inhibitors (9). Also, some melanoma cases present constitutive activation of the PI3K pathway through PTEN loss (20-30% of melanomas) (37). PTEN loss frequently coexists with a BRAF, but not NRAS, mutation. In the case of melanomas with mutated BRAF and loss of PTEN, pharmacological inhibition of BRAF or MEK is less effective than in the case of melanoma without loss of PTEN (38). The combination of BRAF inhibitors with PI3K inhibitors has demonstrated activity in melanomas with PTEN loss, showing increase of BIM expression (37,39,40). Responses to PI3K pathway inhibitors have been reported in BRAF-mutant cancer cell lines, including those without any known PI3K pathway aberration (41).
In addition to mutant BRAF melanomas, inhibition of PI3K also is effective in NRAS mutant melanomas. NRAS mutation simultaneously activates the MAPK and the PI3K pathway (42), so the most logical treatment for RAS mutant tumors would be the combination of drugs, inhibiting both pathways. In mice with KRAS activation, NVP-BEZ235, a dual pan-PI3K/mTOR inhibitor, did not reduced tumor volume, however, when these tumors were treated with the PI3K inhibitor combined with a MEK inhibitor, they underwent regression (43,44). Also, there are multiple feedback loops in the PI3K pathway that can be activated when a PI3K inhibitor is used in monotherapy, so adequate inhibition of this pathway requires the combination of several drugs.
There are many PI3K inhibitors in development, although none have yet been approved. Only mTOR inhibitors have been approved for breast and renal cancer (45-47).
For pan-class I PI3K inhibitors, the importance of the therapeutic window with regard to their pharmacodynamic effect is critical ≥90% inhibition of AKT phosphorylation is necessary to inhibit cancer cell proliferation, implying potentially important differences between the drugs (48).
Buparlisib (BKM120) (Novartis) is an oral pan-class PI3K inhibitor (Figure 1). Preclinical data in murine models demonstrated significant activity in several tumors, including melanoma (49). DLT were hyperglycemia, rash, mood alteration and epigastralgia (50). Combination with the MEK inhibitor trametinib has demonstrated activity in KRAS mutant ovarian cancer (51). Currently, there is an ongoing phase III trial in metastatic breast cancer in combination with hormonotherapy (52). In BRAF mutant melanoma there are ongoing phase I trials in combination with BRAF inhibitors (NCT01512251 and NCT01820364) and MEK inhibitors (NCT013363232).
Pictilisib (GDC-0941) (Genentech) is an oral selective inhibitor of class I PI3K. Since it has been shown to achieve 100% AKT inhibition in two patients at the MTD, it seems a promising inhibitor. It has a good toxicity profile as seen in the first in human trial, and antitumor activity as shown by partial response in two patients. One of whom had V600E BRAF-mutant melanoma; the other had platinum-refractory epithelial ovarian cancer exhibiting PTEN loss and PIK3CA amplification (53). Pictilisib-related hyperglycemia was limited to grades 1-2 elevations, with grade ≥3 hyperglycemia being observed in only one patient (53). In melanoma the combination with cobimetinib (GDC-0973), a MEK inhibitor, demonstrated activity in RAS and BRAF mutant melanoma using intermittent dosing schedule (54).
Other pan-class I PI3K inhibitors that have undergone phase I clinical evaluation include SAR245408 and the irreversible inhibitor wortmannin derivative PX-866 (Oncothyreon). Both drugs were associated with minimal hyperglycemia, but differences were observed in the frequency of rash, which occurred in 26% with SAR245048 and not with PX-866. DLT of PX-866 was diarrhea and elevation of aspartate aminotransferase (55). A phase I trial in melanoma with PX-866 in combination with vemurafenib has been completed (NCT01616199).
A second group of PI3K inhibitors are those that inhibit only some isoforms in a selective way (56). These PI3K inhibitors have limited toxicity even at high doses. In tumors with PI3Kα mutation or those in which PI3K signaling is driven by activated RTKs, PI3Kα is the dominant isoform, whereas in tumors with PTEN mutations, PI3Kβ is dominant (57-59). These data suggest that selective inhibitors of PI3Kβ, but not of PI3Kα, will effectively inhibit PI3K signaling in melanomas in which PTEN is inactivated.
Taselisib (GDC-0032) (Genentech) is a class I selective oral inhibitor of PI3K isoforms-α-δ, and -γ. Its main toxicity is hyperglycemia and asthenia (60). There are ongoing studies in breast cancer, but not in melanoma.
Copanlisib (BAY 80-6946) (Bayer) is a class I selective inhibitor of PI3K isoform-α and β (61) which has shown activity in several types of tumor cell lines, including melanoma (62). A phase I clinical trial has shown good tolerability profile with DLT including left ventricular dysfunction, hyperglycemia, rash, renal and hepatic dysfunction (63). A phase I trial in combination with a MEK inhibitor has been completed (NCT01392521).
BEZ-235 (Novartis) is a dual inhibitor of PI3K (p110 isoforms-α, -β, -γ, -δ) and mTOR with DLT of mucositis (64). Novartis has decided to discontinue its clinical development.
Alpelisib (BYL-719) is an oral selective inhibitor of PI3K isoform-α that is active against the somatic PI3Kα mutations and wild-type PI3Kα (65). Significant anti-tumor activity has been observed in mice bearing PIK3CA-dependent tumor xenograft models and good tolerance (65). DLTs are hyperglycemia and diarrhea (66). Clinical trials ongoing are mainly in breast cancer, but in the phase I study in combination with a MEK inhibitor, melanoma patients were also treated (NCT01449058).
BGT-226 (Novartis) is a dual inhibitor of pan-class I PI3K and mTOR. Its main toxicity is gastrointestinal (nausea and vomiting). It had limited activity in the phase I trial in solid tumors (67). There are no clinical trials ongoing.
GSK-2126458 (GSK) is a selective inhibitor of PI3K isoforms p110-α, -β, -γ, -δ, mTORC1 and mTORC2. The phase I trial of the combination with a MEK inhibitor was stopped due to toxicity and lack of efficacy (NCT01248858).
MK-2206: is a pan-AKT inhibitor (Figure 1). In preclinical studies it has demonstrated synergy with different compounds included chemotherapy (68). A phase I trial has demonstrated tolerability testing different schedules (69). Recently there have been reported two cases with response in wild type BRAF melanoma (70). There are no clinical trials ongoing in melanoma.
AZD8186 (AZ) is a selective inhibitor of PI3K isoforms p110-β and -δ (71). Combined inhibition of both isoforms in PTEN-deficient models, results in major tumor regressions. Combination with inhibition of PI3K isoforms p110-α in prostate model plus hormonotherapy demonstrated high activity (72). It has recently entered phase I clinical trials for PTEN deficient solid malignances (NCT01884285).
Notch pathway inhibitors
The Notch signaling pathway plays a key role in the processes of differentiation and maintenance of cancer stem cells. There are four Notch receptors (Notch 1, 2, 3 and 4) and five ligands (including delta ligand DLL1, DLL3, DLL4 and Jagged JAG 1 and 2). These receptors and their ligands are transmembrane proteins that activate intercellular signaling connections between neighboring cells. Upon binding of the ligand to the receptor, ADAM (also called TACE “metalloproteinase tumor necrosis factor alpha converting enzyme”) is activated. This activation leads to the release of the NICD into the cytoplasm and it translocates to the nucleus where it binds to the transcription factor CSL, activating gene transcription Notch dependent and genes encoding the HES protein include p21, her2, ciclinaD1 and 3, c-myc, neuregulin and some members of NF-κB pathway. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, is in patients with non-small cell lung cancer.
The Notch pathway plays an important role in epithelial-mesenchymal transition (EMT) (73). Recently, cross talk between Notch and Hedgehog pathway have been described since HES1, a target of Notch, directly modulates the transcription factor Gli-155 (74,75).
In solid tumors, although mutations in Notch1 are infrequent, there are frequent alterations in the expression of wild-type Notch receptors and their ligands (76). In melanoma, high levels of HES1 correlate with a poor prognosis (77). Tumors are frequently composed of heterogeneous cell types including cancer stem cells that are resistant to conventional therapies. Therapeutic strategies that effectively target Notch signaling could have a major impact on cancer patient survival by eliminating cancer stem cells. The clinical development of drugs that inhibit the Notch pathway has encountered serious difficulties due to gastrointestinal toxicity. This has been managed by trying discontinuous dosing schedules, concomitant corticosteroid therapy and developing more selective drugs (78).
Drugs targeting the Notch pathway can be generally classified in two groups: γ-secretase inhibitors (GSI) and monoclonal antibodies against the interaction of receptors and ligands of Notch (74).
A wide variety of GSI have entered clinical development; because of the diversity of GSIs and their substrates, the targeting for Notch cleavage is often not highly specific (Figure 1).
MK-0752 (Merck) is a GSI drug. In a phase I study it demonstrated activity in cerebral tumors (79). The daily treatment scheme is highly toxic but the intermittent scheme seems more appropriate (79). Currently there are no studies open in melanoma, although there are in other tumors types such as pancreatic cancer and breast cancer.
RO-4929097 (Roche) is a GSI that in early clinical studies demonstrated activity in several tumor subtypes, including melanoma (80). Initial clinical testing suggested a favorable toxicity profile, with low grade toxicities of fatigue, thrombocytopenia, rash, chills, and anorexia (80). Activity was seen in patients with refractory metastatic or locally advanced solid tumors; tumor responses included one complete response in a patient with melanoma (80). In a phase II study of 33 evaluable patients with metastatic colorectal cancer, there were no objective radiographic responses and six patients had stable disease (81). Development of RO4929097 has been discontinued.
PF-03084014 (Pfizer) is a non-competitive reversible GSI which has shown activity in triple-negative breast cancer and desmoid tumors in a phase I study (82). There are no ongoing specific studies in melanoma.
Other GSI in early clinical development are: BMS-708 163, BMS-906 024, LY3039478, LY900009 and MK-0752.
The monoclonal antibodies against receptor ligands of Notch are in early stages of clinical development in various tumor types (though not specifically in melanoma): OMP-59R5 (tarextumab), OMP-21M18 (demcizumab), REGN421/SAR153192, OMP-52M51 (83).
TGF-β pathway inhibitors
TGF-β has biphasic effects during tumorigenesis. In early stages of tumor development, it acts as a tumor suppressor but in the later stages it contributes to its development, inducing EMT (84).
EMT is a dynamic process in which cells undergo bidirectional differentiation from epithelial to mesenchymal state. Cells in different stages of epithelial-mesenchymal differentiation usually co-exist within the same tumor. Cells with mesenchymal differentiation are more invasive. EMT is associated with the acquisition of properties of cancer stem cells and, therefore, worse response to treatment. TGF-β is an important signaling pathway involved in the EMT phenomenon, and a number of transcription factors such as Twist, Snail, Slug and ZEB1 are involved in gene expression changes associated with the mesenchymal phenotype (85). In melanoma, transcription factors ZEB2 and Snail 2 are expressed in normal melanocytes and act as tumor suppressors to activate the program of melanocytic differentiation dependent on MITF. During tumor progression there is an increase in expression of ZEB1 and TWIST1 (86). Melanomas with EMT activation have low levels of MITF.
EMT activation also makes tumors resistant to immunotherapy. Therapeutic strategies have been proposed in laboratory models inducing melanoma differentiation, which reinstates MITF and reverses resistance to immunotherapy (87).
TGF-β ligands (TGF-β1, TGF-β2, TGF-β3) bind the transmembrane serine/threonine kinase TβRI (ALK5), which in turn activate the receptor type II. The heterodimer complex (TβRI with TβRII) phosphorylates the intracellular proteins SMAD2 and downstream effectors SMAD3, SMAD4 that activate signaling to induce several nuclear transduction proteins (88).
In addition, TGF-β is a potent immunosuppressive cytokine that inhibits T cell function and antigen presenting capacity of dendritic cells. In melanoma murine models immunoevasion mechanisms increasing TGF-β in the population of dendritic cells and upregulation of the enzyme IDO in dendritic cells have been described (89,90).
Many tumors have overexpression of TGF-β. In advanced melanoma overexpression of TGF-β1, TGF-β2 and TGF-β3 is common. Constitutive SMAD signal occurs in response to autocrine secretion of TGF-β. GLI2, a transcription factor involved in the Hedgehog pathway and a transcriptional target of the TGF-β/SMAD, is overexpressed in invasive melanoma (91).
There are several different types of TGF-β inhibitors: small molecule inhibitors of TGF-β receptor type I and II, monoclonal antibodies against TGF-β ligands, and antisense oligonucleotides that block production of TGF-β ligands (Figure 1). However, none of these treatments has yet achieved approval for clinical use (92,93).
Trabedersen (AP12009) is an antisense molecule against TGF-β2 which has been studied in phase I-II clinical trials in several tumors, demonstrating a good toxicity profile. In melanoma patients it demonstrated activity with a median survival close to 1 year (94). There are no clinical trials ongoing.
Fresolimumab (GC1008) (Genzyme) is a monoclonal antibody against anti-TGF-β that neutralizes all isoforms of TGF-β. A phase I study has shown activity in patients with melanoma, with good tolerability. The development of reversible cutaneous keratoacanthomas/squamous-cell carcinomas and hyperkeratosis was the major adverse event (95). There is currently an open phase I trial in combination with radiation therapy in breast cancer that has recently completed recruitment (NCT01401062). There are no ongoing studies in melanoma.
Other antibodies developed by the same pharmaceutical company are CAT-152 (Lerdelimimab) and CAT-192 (Metelimumab), which inhibit TGF-β1 and TGF-β2 respectively.
PF-03446962 (Pfizer) is a humanized monoclonal antibody against ALK-1 (a specific receptor of TGF-β). It has demonstrated safety and but limited clinical activity in phase I-II studies in solid tumors (96,97). There is only one clinical trial ongoing in colorectal cancer.
SB-431542 and SB-505124 (GSK) are small molecule inhibitors of ALK5. They inhibit angiogenesis, and increase maturation of dendritic cell activity and CD8 T cells (98-100). No clinical studies are underway.
YR-290 is a tyrosine kinase inhibitor of ALK5 that has demonstrated activity in breast cancer in animal models (101).
Tasisulam (LY573636) is a tyrosine kinase inhibitor of TGF-β/ALK5. In melanoma, a phase III trial vs. chemotherapy was closed early due to toxicity, with three deaths related to the drug (thrombocytopenia) and no activity compared with chemotherapy (102).
EW-7203, 7195-EW and EW-7197 are potent oral inhibitors of TGF-β/ALK5 (103-106), which have shown activity in animal models of melanoma (106).
Galunisertib (LY2157299) (Lilly) is a small molecule inhibitor of ALK5. As with other ALK5 inhibitors, it is not only blocks the phosphorylation of R-Smads, but also induces degradation of Smad4, mainly CD8+ T cells, and increases anti-melanoma CTL response (106). It is the first small molecule inhibitor that has passed to advanced clinical development. In the first in-human trial, it has demonstrated tolerability and preliminary activity in gliomas (107). It has been tested in combination with TKIs such as gefitinib or crizotinib in tumors with MED12 loss (MED12 loss produces activation of TGF-β and activates the EMT process), demonstrating suppression of ERK. It has been suggested that this combination could be active in tumors with high TGF-β signal (108). Currently there is an ongoing phase I study of LY2157299 in combination with the anti PD-1 antibody nivolumab in solid tumors (NCT02423343).
Other drugs from this group are in preclinical development: KI26894, LY364937, LY2109761, SD093, SD208 and LY580276.
Inhibitors of YAP-TEAD1: the Hippo pathway
The Hippo pathway maintains the transcriptional activators Yes-associated protein (YAP) and TAZ in phosphorylated forms in the cytoplasm, preventing cell proliferation. When the Hippo pathway is inactivated, YAP and TAZ are translocated to the nucleus and induce the expression of a variety of proteins concerned with entry into the cell division cycle, such as cyclin D1 and Fox M1, as well as the inhibition of apoptosis (109). Involvement of Hippo pathway in the development of melanoma has been demonstrated, activating processes of invasion, metastasis and resistance (109,110).
Verteporfin inhibits the assembly of a functional YAP-TEAD transcription factor and has been developed mainly in ophthalmology for the treatment of macular degeneration. A phase I-II clinical trial in advanced melanoma was conducted with photodynamic therapy (NCT00007969).
Heat shock protein 90 (Hsp90) inhibitors
Hsp90 is a cellular chaperone that stabilizes and activates over 200 proteins known as “HSP90 clients”. Hsp90 is acetylated by HDAC6 and HDAC, resulting in protein degradation by the proteasome “clients” of Hsp90 as Erb1, Erb2, AKT, CRAF, Flt-3 or BRAF mutant. Hsp90 is necessary for the maturation and function of many cellular proteins, including some that are involved in the pathogenesis of melanoma such as BRAF, CRAF, IGF1R, cyclin D1, CDK4 and AKT (111). Also, tumor cells use Hsp90 as a biochemical buffer to protect their mutated oncoproteins from degradation via the proteasome, thereby facilitating tumor survival driven by an oncogene. Overexpression of Hsp90 in tumors has been associated with drug resistance and poor prognosis (112). Hsp90 inhibition may be especially interesting in resistant BRAF mutant melanoma, as preclinical studies indicate that resistance to BRAF inhibitors when mediated by overexpression COT could be reversed by treatment with Hsp90 inhibitors (113).
The first inhibitor developed was tanespimycin (17-AAG), an analog of geldanamycin from BMS. In a phase II study with 17 patients with metastatic melanoma, there was not observed significant activity (114).
BIIB021 is an oral inhibitor of Hsp90. The phase I study showed acceptable tolerance, with the most frequent toxicity being gastrointestinal. Stabilizations were observed in 11% of cases, none in patients with melanoma (115,116). There are no ongoing studies with this drug in melanoma.
Luminespib (AUY922) is an inhibitor of geldanamycin second generation non-isoxazole Hsp90. In the phase I study in more than 100 patients with various solid tumors there were no objective responses, although it had a good toxicity profile and stabilizations (117). Ongoing clinical studies are focusing on lung cancer.
SNX-5422 in the phase I study treatment-related adverse events were mainly low grade, including diarrhea and nausea. Reversible grade 1-3 nyctalopia (night blindness) was reported by four patients. Objective responses in breast cancer and prostate cancer patients were observed (118).
Ganetespib (STA-9090) is active in resistant BRAF mutant melanoma cell lines with loss of PTEN, inactivation of RB1 or overexpression of COT (111,119). Ganetespib also shows greater activity than AZD6244 or vemurafenib against melanoma cell lines with BRAF mutation. The combination of ganetespib with a MEK inhibitor in BRAF resistant melanomas is active in preclinical models (120). Two phase II clinical trials have been completed in advanced melanoma, as well as in ocular and cutaneous melanoma (NCT01200238, NCT01551693).
XL888 has demonstrated activity in resistant BRAF mutant melanoma cell lines and animal models (121). Currently there is a phase I study in melanoma combining XL888 and vemurafenib (NCT01657591).
Inhibitors of growth factors and their receptors
Development of adaptive resistance with a rapid overexpression of RTKs and/or their stimulating factors as PDGFBR, IGFR1, hepatocyte growth factor (HGF)/c-MET, EGFR3/neuroregulin, FGFR1-4/FGF2 and AXL/Gas6 is common during treatment of BRAF mutant melanomas with BRAF and MEK inhibitors (28). This resistance is mediated by overexpression of RTKs and could be inhibited by combining BRAF inhibitors with RTK inhibitors. In BRAF mutant melanomas, the production of HGF confers resistance to vemurafenib (122); in NRAS mutant melanomas the role of MET/HGF is well known. Recently, six different melanocytic tumors with genomic rearrangements of MET fusing the kinase domain of MET in-frame to six different N-terminal partners have been identified (123). Some MET receptor tyrosine kinase inhibitors are in clinical development.
Tivantinib (ARQ197) in combination with sorafenib has shown clinical activity in a phase I trial with two partial responses and four stabilizations in six patients with NRAS mutant melanomas. Two phase III trials in NSCLC have recently reported an improvement in DFS (124,125).
PHA665752 reduces the proliferation and migration of NRAS mutant cell lines (126).
Onartuzumab (Roche) is a monoclonal antibody against MET that inhibits the binding of HGF to its receptor (127). Recruitment has been recently completed for a phase I clinical trial of onartuzumab in combination with cobimetinib and vemurafenib (NCT01974258).
Crizotinib (Pfizer) is an inhibitor of c-MET, ROS1 and ALK. In melanoma activity in preclinical studies in uveal melanoma has been demonstrated (128). A phase II trial is currently recruiting patients with uveal melanoma after definitive adjuvant treatment with crizotinib (NCT02223819).
E7050 (Eisai) is an inhibitor of c-MET and VEGFR2 (129). A phase II clinical trial in combination with E7080 in advanced melanoma has recently completed recruitment (NCT01433991). Other RTKs involved in melanoma progression and resistance, are PDGFR, IGFR, EGFR, FGFR and Erb3.
Ganitumab (AMG479) (Amgen) is a IGFR1 inhibitor that has demonstrated activity in melanoma cell lines (130). The combination of a MEK inhibitor with a IGF1R inhibitor demonstrated activity in preclinical models (131). In melanoma patients, a phase I study in combination with MEK162 has been completed (NCT01562899).
MEHD7945A (Genentech) is a monoclonal bispecific antibody that inhibits EGFR and HER3. Melanoma cells often express HER3 and neuroregulin which, acting through HER3 activates the PI3K/AKT pathway. Erb3 is overexpressed in BRAF mutant melanomas in response to inhibition of the MAPK pathway (132). MEHD7945A has demonstrated activity in EGFR inhibitor resistant tumors but has not been studied in melanoma (133,134). A phase I trial in solid tumors demonstrated a good toxicity profile with grade 3 toxicity only in one patient with diarrhea and nausea (135). Clinical trials were focused mainly on squamous head and neck and colorectal cancer due to demonstrated activity in these tumors (135). MEHD7945A is currently being studied in a phase I clinical trial in KRAS mutant tumors in combination with cobimetinib, a MEK inhibitor (NCT01986166).
Other EGFR inhibitors have shown activity in experiments with cell lines and mouse models in melanoma. Lapatinib, a TKI of EGFR and HER2, in combination with BRAF inhibitor reverse resistance in BRAF mutant melanomas (136,137). A phase II trial in Erb4 mutant melanoma patients has been terminated and no clinical responses observed (NCT01264081).
Expression in melanoma cell lines of FGFR1, FGFR4 and FGF family ligands is particularly high. Pharmacological inhibition of FGFRs restores sensitivity in melanoma cells resistant to vemurafenib (138). There are many FGFR inhibitors in development. Among non-selective FGFR TKI are Dovitinib (TKI258), Lucitanib (E3810), Nintedanib (BIBF1120), Brivanib (BMS582664), Lenvatinib (E7080), Ponatinib (AP24534), Orantinib (TSU68) and ENMD-2076. Selective FGFR TKI are BJG398, AZD4547, Debio1347, LY287445, ARQ087, JNJ-42756493 and TAS120 Also there are antibodies and FGF-ligand TRAPas MGFR1877S (RG744) and FP-1039 (GSK3052230) (139). In melanoma models, SU5402, a small molecule FGFR inhibitor, has demonstrated activity (140). In melanoma patients the panFGFR inhibitor BGJ398 (Novartis) is being tested in combination with the BRAF and MEK inhibitor in a phase I trial (NCT02159066) (Figure 1).
Other targeted drugs
Among multikinase inhibitors Pazopanib is an inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFRβ, PDGFRα and c-kit that has FDA approval for sarcoma and renal cell carcinoma. In the phase I trial the most common toxicities were alopecia, fatigue, hypertension, nausea, diarrhea, dysgeusia, neutropenia, myalgia, hair color changes, and peripheral neuropathy, with a manageable safety profile (141). Responses have been reported in melanoma when combining with chemotherapy (141,142). Pazopanib is being tested in a phase II trial in melanoma in combination with chemotherapy (NCT01107665).
Cabozantinib (Exelixis) is a multikinase inhibitor of MET, VEGFR2, RET, KIT, AXL and FLT3, FDA-approved for progressive medullary thyroid cancer. It is active in melanomas with MET fusion proteins (TRIM4-MET and ZKSCAN1-MET) (123). Cabozantinib demonstrated 5% of objective responses in a phase II trial in cutaneous and ocular melanoma (143). A trial comparing with chemotherapy in ocular melanoma is ongoing (NCT 01835145) and a phase I trial in combination with the MEK inhibitor MEK162 has been terminated (NCT01835184).
RAF265 (Novartis) is an inhibitor of wild type and mutant BRAF, VEGFR2, CRAF, PDGFR, CSF, RET, ckit and SRC. It demonstrated activity in the first in-human study both in BRAF mutant and wild type tumors (144). A phase II trial in melanoma has been completed testing different schedules (NCT 00304525).
JAK/STAT inhibitors are under development in multiple tumors including melanoma. WP1066 is a STAT3 inhibitor with activity in resistant BRAF melanoma, there is an ongoing clinical trial in melanoma patients with brain metastases (NCT 01904123) (Figure 1). Melanoma cells are sensitive to other STAT3 inhibitors such as curcumin and their analogues, and to other natural inhibitors such as BBMD3, a derivative of the natural bis-benzylisoquinoline alkaloid berberine (145).
Other therapeutic targets in melanoma are have been recently defined, such as IL-2 inducible T-cell kinase (ITK), a known immune cell-specific protein I is inhibited by the small-molecule inhibitor BI 10N that is active in mouse melanoma models (146).
Conclusions
In recent years the clinical development and approval of BRAF and MEK inhibitors for treatment of BRAF mutant melanoma has led to increased interest in drug discovery for molecular targets implicated in melanoma development and progression.
Although the main focus of interest in this area has moved towards immunotherapy, several new drugs that inhibit the main activated pathways in melanoma are now in clinical development. PanRAF inhibitors and ERK inhibitors could be of special interest for treatment of BRAF mutant melanoma as they can circumvent the main resistance mechanisms to BRAF inhibitors. Evidence precludes the development of several combinations as MAPK inhibitors with PI3K inhibitors or RTK inhibitors, as TGFR inhibitors with immunotherapy. Ever-growing understanding of melanoma molecular biology will help us design the most active and specific drugs for continued improvements in treatment in the coming years.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527-34. [PubMed]
- Karachaliou N, Molina-Vila MA, Rosell R. The impact of rare EGFR mutations on the treatment response of patients with non-small cell lung cancer. Expert Rev Respir Med 2015;9:241-4. [PubMed]
- Garcia del Muro X. Nilotinib, imatinib, and GIST therapy. Lancet Oncol 2015;16:483-4. [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [PubMed]
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015;386:444-51. [PubMed]
- Meier F, Schittek B, Busch S, et al. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci 2005;10:2986-3001. [PubMed]
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80-93. [PubMed]
- Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011;480:387-90. [PubMed]
- Lidsky M, Antoun G, Speicher P, et al. Mitogen-activated protein kinase (MAPK) hyperactivation and enhanced NRAS expression drive acquired vemurafenib resistance in V600E BRAF melanoma cells. J Biol Chem 2014;289:27714-26. [PubMed]
- Sievert AJ, Lang SS, Boucher KL, et al. Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas. Proc Natl Acad Sci USA 2013;110:5957-62. [PubMed]
- Nakamura A, Arita T, Tsuchiya S, et al. Antitumor activity of the selective pan-RAF inhibitor TAK-632 in BRAF inhibitor-resistant melanoma. Cancer Res 2013;73:7043-55. [PubMed]
- Okaniwa M, Hirose M, Arita T, et al. Discovery of a selective kinase inhibitor (TAK-632) targeting pan-RAF inhibition: design, synthesis, and biological evaluation of C-7-substituted 1,3-benzothiazole derivatives. J Med Chem 2013;56:6478-94. [PubMed]
- Girotti MR, Lopes F, Preece N, et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell 2015;27:85-96. [PubMed]
- Wong DJ, Robert L, Atefi MS, et al. Antitumor activity of the ERK inhibitor SCH722984 against BRAF mutant, NRAS mutant and wild-type melanoma. Mol Cancer 2014;13:194. [PubMed]
- Morris EJ, Jha S, Restaino CR, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer discovery 2013;3:742-50. [PubMed]
- Das Thakur M, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013;494:251-5. [PubMed]
- Wang AX, Qi XY. Targeting RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB life 2013;65:748-58. [PubMed]
- Whittaker SR, Theurillat JP, Van Allen E, et al. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov 2013;3:350-62. [PubMed]
- Patnaik S, Dietz HC, Zheng W, et al. Multi-gram scale synthesis of FR180204. J Org Chem 2009;74:8870-3. [PubMed]
- Robarge K, Schwarz J, Blake J, et al. Abstract DDT02-03: Discovery of GDC-0994, a potent and selective ERK1/2 inhibitor in early clinical development. Cancer Res 2014;74:DDT02-3.
- Sheppard KE, McArthur GA. The cell-cycle regulator CDK4: an emerging therapeutic target in melanoma. Clin Cancer Res 2013;19:5320-8. [PubMed]
- Marian C, Scope A, Laud K, et al. Search for germline alterations in CDKN2A/ARF and CDK4 of 42 Jewish melanoma families with or without neural system tumours. Br J Cancer 2005;92:2278-85. [PubMed]
- Du J, Widlund HR, Horstmann MA, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 2004;6:565-76. [PubMed]
- DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 2015;21:995-1001. [PubMed]
- Turner NC, Ro J, Andre F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med 2015;373:209-19. [PubMed]
- Kwong LN, Davies MA. Targeted therapy for melanoma: rational combinatorial approaches. Oncogene 2014;33:1-9. [PubMed]
- Burdette-Radoux S, Tozer RG, Lohmann RC, et al. Phase II trial of flavopiridol, a cyclin dependent kinase inhibitor, in untreated metastatic malignant melanoma. Invest New Drugs 2004;22:315-22. [PubMed]
- Desai BM, Villanueva J, Nguyen TT, et al. The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling. PLoS ONE 2013;8:e59588. [PubMed]
- Kumar SK, LaPlant B, Chng WJ, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 2015;125:443-8. [PubMed]
- Sosman JA, Kittaneh M, Lolkema MPJK, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J Clin Oncol 2014;32:abstr 9009.
- Yeo W, Goh B, Le Tourneau C, et al. A phase II randomized study of oral seliciclib in patients with previously treated nasopharyngeal carcinoma. J Clin Oncol 2009;27:abstr 6026.
- Patnaik A, Rosen LS, Tolaney SM, et al. Abstract CT232: Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with metastatic breast cancer. Cancer Res 2014;74:CT232.
- Goldman JW, Gandhi L, Patnaik A, et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with non-small cell lung cancer. J Clin Oncol 2014;32:abstr 8026.
- Davies MA. The role of the PI3K-AKT pathway in melanoma. Cancer J 2012;18:142-7. [PubMed]
- Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011;71:2750-60. [PubMed]
- Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 2013;31:1767-74. [PubMed]
- Sánchez-Hernández I, Baquero P, Calleros L, et al. Dual inhibition of (V600E)BRAF and the PI3K/AKT/mTOR pathway cooperates to induce apoptosis in melanoma cells through a MEK-independent mechanism. Cancer Lett 2012;314:244-55. [PubMed]
- Sweetlove M, Wrightson E, Kolekar S, et al. Inhibitors of pan-PI3K Signaling Synergize with BRAF or MEK Inhibitors to Prevent BRAF-Mutant Melanoma Cell Growth. Frontiers in oncology 2015;5:135. [PubMed]
- Lassen A, Atefi M, Robert L, et al. Effects of AKT inhibitor therapy in response and resistance to BRAF inhibition in melanoma. Mol Cancer 2014;13:83. [PubMed]
- Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther 2012;11:909-20. [PubMed]
- Roberts PJ, Usary JE, Darr DB, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res 2012;18:5290-303. [PubMed]
- Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nature medicine 2008;14:1351-6. [PubMed]
- Tartarone A, Lerose R, Aieta M. Everolimus in HR-positive advanced breast cancer. N Engl J Med 2012;366:1739; author reply 1739-40.
- Massarweh S, Croley J, Weiss H. Everolimus in HR-positive advanced breast cancer. N Engl J Med 2012;366:1738-9; author reply 1739-40.
- Czarnecka AM, Kornakiewicz A, Lian F, et al. Future perspectives for mTOR inhibitors in renal cell cancer treatment. Future Oncol 2015;11:801-17. [PubMed]
- Raynaud FI, Eccles SA, Patel S, et al. Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther 2009;8:1725-38. [PubMed]
- Koul D, Fu J, Shen R, et al. Antitumor activity of NVP-BKM120--a selective pan class I PI3 kinase inhibitor showed differential forms of cell death based on p53 status of glioma cells. Clin Cancer Res 2012;18:184-95. [PubMed]
- Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol 2012;30:282-90. [PubMed]
- Bedard PL, Tabernero J, Janku F, et al. A phase Ib dose-escalation study of the oral pan-PI3K inhibitor buparlisib (BKM120) in combination with the oral MEK1/2 inhibitor trametinib (GSK1120212) in patients with selected advanced solid tumors. Clin Cancer Res 2015;21:730-8. [PubMed]
- Buparlisib and letrozole have activity in metastatic breast cancer. Cancer Discov 2014;4:507. [PubMed]
- Sarker D, Ang JE, Baird R, et al. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2015;21:77-86. [PubMed]
- Shapiro G, LoRusso P, Kwak EL, et al. Clinical combination of the MEK inhibitor GDC-0973 and the PI3K inhibitor GDC-0941: A first-in-human phase Ib study testing daily and intermittent dosing schedules in patients with advanced solid tumors. J Clin Oncol 2011;29:abstr 3005.
- Hong DS, Bowles DW, Falchook GS, et al. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res 2012;18:4173-82. [PubMed]
- Juric D, Rodon J, Gonzalez-Angulo AM, et al. Abstract CT-01: BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Res 2012;72:CT-01.
- Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 2006;125:733-47. [PubMed]
- Ni J, Liu Q, Xie S, et al. Functional characterization of an isoform-selective inhibitor of PI3K-p110beta as a potential anticancer agent. Cancer Discov 2012;2:425-33. [PubMed]
- Wee S, Wiederschain D, Maira SM, et al. PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 2008;105:13057-62. [PubMed]
- Juric D, Krop I, Ramanathan RK, et al. GDC-0032, a Beta Isoform-Sparing PI3K Inhibitor: Results of a First-in-Human Phase 1ª Dose Escalation Study. Cancer Res 2013;73:abs LB-64.
- Liu N, Rowley BR, Bull CO, et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110alpha and p110delta activities in tumor cell lines and xenograft models. Mol Cancer Ther 2013;12:2319-30. [PubMed]
- Schneider P, Schon M, Pletz N, et al. The novel PI3 kinase inhibitor, BAY 80-6946, impairs melanoma growth in vivo and in vitro. Exp Dermatol 2014;23:579-84. [PubMed]
- Patnaik A, Appleman LJ, Mountz JM, et al. A first-in-human phase I study of intravenous PI3K inhibitor BAY 80-6946 in patients with advanced solid tumors: Results of dose-escalation phase. J Clin Oncol 2011;29:abstr 3035.
- Bendell JC, Kurkjian C, Infante JR, et al. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Invest New Drugs 2015;33:463-71. [PubMed]
- Fritsch C, Huang A, Chatenay-Rivauday C, et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol Cancer Ther 2014;13:1117-29. [PubMed]
- Juric D, Argiles G, Burris HA, et al. Abstract P6-10-07: Phase I study of BYL719, an alpha-specific PI3K inhibitor, in patients with PIK3CA mutant advanced solid tumors: preliminary efficacy and safety in patients with PIK3CA mutant ER-positive (ER+) metastatic breast cancer (MBC). Cancer Res 2012;72:P6-10-07.
- Markman B, Tabernero J, Krop I, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of the oral phosphatidylinositol-3-kinase and mTOR inhibitor BGT226 in patients with advanced solid tumors. Ann Oncol 2012;23:2399-408. [PubMed]
- Hirai H, Sootome H, Nakatsuru Y, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 2010;9:1956-67. [PubMed]
- Yap TA, Yan L, Patnaik A, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res 2014;20:5672-85. [PubMed]
- Rebecca VW, Massaro RR, Fedorenko IV, et al. Inhibition of autophagy enhances the effects of the AKT inhibitor MK-2206 when combined with paclitaxel and carboplatin in BRAF wild-type melanoma. Pigment Cell Melanoma Res 2014;27:465-78. [PubMed]
- Barlaam B, Cosulich S, Degorce S, et al. Discovery of (R)-8-(1-(3,5-difluorophenylamino)ethyl)-N,N-dimethyl-2-morpholino-4-oxo-4H-chrom ene-6-carboxamide (AZD8186): a potent and selective inhibitor of PI3Kbeta and PI3Kdelta for the treatment of PTEN-deficient cancers. J Med Chem 2015;58:943-62. [PubMed]
- Schwartz S, Wongvipat J, Trigwell CB, et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer cell 2015;27:109-22. [PubMed]
- Li Y, Ma J, Qian X, et al. Regulation of EMT by Notch signaling pathway in tumor progression. Curr Cancer Drug Targets 2013;13:957-62. [PubMed]
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015;12:445-64. [PubMed]
- Schreck KC, Taylor P, Marchionni L, et al. The Notch target Hes1 directly modulates Gli1 expression and Hedgehog signaling: a potential mechanism of therapeutic resistance. Clin Cancer Res 2010;16:6060-70. [PubMed]
- Bedogni B. Notch signaling in melanoma: interacting pathways and stromal influences that enhance Notch targeting. Pigment Cell Melanoma Res 2014;27:162-8. [PubMed]
- Huynh C, Poliseno L, Segura MF, et al. The novel gamma secretase inhibitor RO4929097 reduces the tumor initiating potential of melanoma. PLoS ONE 2011;6:e25264. [PubMed]
- Grosveld GC. Gamma-secretase inhibitors: Notch so bad. Nat Med 2009;15:20-1. [PubMed]
- Krop I, Demuth T, Guthrie T, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 2012;30:2307-13. [PubMed]
- Tolcher AW, Messersmith WA, Mikulski SM, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 2012;30:2348-53. [PubMed]
- Strosberg JR, Yeatman T, Weber J, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer 2012;48:997-1003. [PubMed]
- Zhang CC, Yan Z, Zong Q, et al. Synergistic effect of the gamma-secretase inhibitor PF-03084014 and docetaxel in breast cancer models. Stem Cells Transl Med 2013;2:233-42. [PubMed]
- Yen WC, Fischer MM, Axelrod F, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res 2015;21:2084-95. [PubMed]
- Smith AL, Robin TP, Ford HL. Molecular pathways: targeting the TGF-beta pathway for cancer therapy. Clin Cancer Res 2012;18:4514-21. [PubMed]
- Yu H, Shen Y, Hong J, et al. The contribution of TGF-beta in Epithelial-Mesenchymal Transition (EMT): Down-regulation of E-cadherin via snail. Neoplasma 2015;62:1-15. [PubMed]
- Caramel J, Papadogeorgakis E, Hill L, et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 2013;24:466-80. [PubMed]
- Sáez-Ayala M, Montenegro MF, Sánchez-Del-Campo L, et al. Directed phenotype switching as an effective antimelanoma strategy. Cancer Cell 2013;24:105-19. [PubMed]
- Gordon KJ, Blobe GC. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 2008;1782:197-228.
- Lin C, Lin CN, Wang YC, et al. The role of TGF-beta signaling and apoptosis in innate and adaptive immunity in zebrafish: a systems biology approach. BMC systems biology 2014;8:116. [PubMed]
- Taylor AW. Review of the activation of TGF-beta in immunity. Journal of leukocyte biology 2009;85:29-33. [PubMed]
- Javelaud D, van Kempen L, Alexaki VI, et al. Efficient TGF-beta/SMAD signaling in human melanoma cells associated with high c-SKI/SnoN expression. Mol Cancer 2011;10:2. [PubMed]
- Ling LE, Lee WC. Tgf-beta type I receptor (Alk5) kinase inhibitors in oncology. Current pharmaceutical biotechnology 2011;12:2190-202. [PubMed]
- Lahn M, Kloeker S, Berry BS. TGF-beta inhibitors for the treatment of cancer. Expert Opin Investig Drugs 2005;14:629-43. [PubMed]
- Oettle H, Seufferlein T, Luger T, et al. Final results of a phase I/II study in patients with pancreatic cancer, malignant melanoma, and colorectal carcinoma with trabedersen. J Clin Oncol 2012;30:abstr 4034.
- Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS ONE 2014;9:e90353. [PubMed]
- Zucali PA, Thomas MB, et al. Phase I study of PF-03446962 (anti-ALK-1 mAb) in hepatocellular carcinoma (HCC). J Clin Oncol 2013;31:abstr 4121.
- Necchi A, Giannatempo P, Mariani L, et al. PF-03446962, a fully-human monoclonal antibody against transforming growth-factor beta (TGFbeta) receptor ALK1, in pre-treated patients with urothelial cancer: an open label, single-group, phase 2 trial. Invest New Drugs 2014;32:555-60. [PubMed]
- Tanaka H, Shinto O, Yashiro M, et al. Transforming growth factor beta signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol Rep 2010;24:1637-43. [PubMed]
- Halder SK, Beauchamp RD, Datta PK. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia 2005;7:509-21. [PubMed]
- DaCosta Byfield S, Major C, Laping NJ, et al. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol 2004;65:744-52. [PubMed]
- Fang Y, Chen Y, Yu L, et al. Inhibition of breast cancer metastases by a novel inhibitor of TGFbeta receptor 1. J Natl Cancer Inst 2013;105:47-58. [PubMed]
- Hamid O, Ilaria R Jr, Garbe C, et al. A randomized, open-label clinical trial of tasisulam sodium versus paclitaxel as second-line treatment in patients with metastatic melanoma. Cancer 2014;120:2016-24. [PubMed]
- Park CY, Kim DK, Sheen YY. EW-7203, a novel small molecule inhibitor of transforming growth factor-beta (TGF-beta) type I receptor/activin receptor-like kinase-5, blocks TGF-beta1-mediated epithelial-to-mesenchymal transition in mammary epithelial cells. Cancer Sci 2011;102:1889-96. [PubMed]
- Park CY, Son JY, Jin CH, et al. EW-7195, a novel inhibitor of ALK5 kinase inhibits EMT and breast cancer metastasis to lung. Eur J Cancer 2011;47:2642-53. [PubMed]
- Son JY, Park SY, Kim SJ, et al. EW-7197, a novel ALK-5 kinase inhibitor, potently inhibits breast to lung metastasis. Mol Cancer Ther 2014;13:1704-16. [PubMed]
- Yoon JH, Jung SM, Park SH, et al. Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Mol Med 2013;5:1720-39. [PubMed]
- Rodón J, Carducci M, Sepulveda-Sánchez JM, et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest New Drugs 2015;33:357-70. [PubMed]
- Huang S, Holzel M, Knijnenburg T, et al. MED12 controls the response to multiple cancer drugs through regulation of TGF-beta receptor signaling. Cell 2012;151:937-50. [PubMed]
- Kim JE, Finlay GJ, Baguley BC. The role of the hippo pathway in melanocytes and melanoma. Front Oncol 2013;3:123. [PubMed]
- Lin L, Sabnis AJ, Chan E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet 2015;47:250-6. [PubMed]
- Acquaviva J, Smith DL, Jimenez JP, et al. Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib. Mol Cancer Ther 2014;13:353-63. [PubMed]
- Trepel J, Mollapour M, Giaccone G, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 2010;10:537-49. [PubMed]
- Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 2008;68:4853-61. [PubMed]
- Pacey S, Gore M, Chao D, et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs 2012;30:341-9. [PubMed]
- Saif MW, Takimoto C, Mita M, et al. A phase 1, dose-escalation, pharmacokinetic and pharmacodynamic study of BIIB021 administered orally in patients with advanced solid tumors. Clin Cancer Res 2014;20:445-55. [PubMed]
- Dickson MA, Okuno SH, Keohan ML, et al. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann Oncol 2013;24:252-7. [PubMed]
- Sessa C, Shapiro GI, Bhalla KN, et al. First-in-human phase I dose-escalation study of the HSP90 inhibitor AUY922 in patients with advanced solid tumors. Clin Cancer Res 2013;19:3671-80. [PubMed]
- Infante JR, Weiss GJ, Jones S, et al. Phase I dose-escalation studies of SNX-5422, an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumours. Eur J Cancer 2014;50:2897-904. [PubMed]
- Wu X, Marmarelis ME, Hodi FS. Activity of the heat shock protein 90 inhibitor ganetespib in melanoma. PLoS ONE 2013;8:e56134. [PubMed]
- Goldman JW, Raju RN, Gordon GA, et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer 2013;13:152. [PubMed]
- Paraiso KH, Haarberg HE, Wood E, et al. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clin Cancer Res 2012;18:2502-14. [PubMed]
- Matsumura A, Kubota T, Taiyoh H, et al. HGF regulates VEGF expression via the c-Met receptor downstream pathways, PI3K/Akt, MAPK and STAT3, in CT26 murine cells. Int J Oncol 2013;42:535-42. [PubMed]
- Yeh I, Botton T, Talevich E, et al. Activating MET kinase rearrangements in melanoma and Spitz tumours. Nat Commun 2015;6:7174. [PubMed]
- Scagliotti G, von Pawel J, Novello S, et al. Phase III Multinational, Randomized, Double-Blind, Placebo-Controlled Study of Tivantinib (ARQ 197) Plus Erlotinib Versus Erlotinib Alone in Previously Treated Patients With Locally Advanced or Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2667-74. [PubMed]
- Yoshioka H, Azuma K, Yamamoto N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol 2015;26:2066-72. [PubMed]
- Chattopadhyay C, Ellerhorst JA, Ekmekcioglu S, et al. Association of activated c-Met with NRAS-mutated human melanomas. Int J Cancer 2012;131:E56-65. [PubMed]
- Xiang H, Bender BC, Reyes AE 2nd, et al. Onartuzumab (MetMAb): using nonclinical pharmacokinetic and concentration-effect data to support clinical development. Clin Cancer Res 2013;19:5068-78. [PubMed]
- Surriga O, Rajasekhar VK, Ambrosini G, et al. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol Cancer Ther 2013;12:2817-26. [PubMed]
- Wang W, Li Q, Takeuchi S, et al. Met kinase inhibitor E7050 reverses three different mechanisms of hepatocyte growth factor-induced tyrosine kinase inhibitor resistance in EGFR mutant lung cancer. Clin Cancer Res 2012;18:1663-71. [PubMed]
- Euw EMV, Konkankit VV, Gong KW, et al. Abstract LB-257: Ganitumab (AMG 479), an anti IGF-1R antibody, inhibits the proliferation of melanoma cells. Cancer Res 2012;72:LB-257.
- Villanueva J, Vultur A, Herlyn M. Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res 2011;71:7137-40. [PubMed]
- Fattore L, Marra E, Pisanu ME, et al. Activation of an early feedback survival loop involving phospho-ErbB3 is a general response of melanoma cells to RAF/MEK inhibition and is abrogated by anti-ErbB3 antibodies. J Transl Med 2013;11:180. [PubMed]
- Kamath AV, Lu D, Gupta P, et al. Preclinical pharmacokinetics of MEHD7945A, a novel EGFR/HER3 dual-action antibody, and prediction of its human pharmacokinetics and efficacious clinical dose. Cancer Chemother Pharmacol 2012;69:1063-9. [PubMed]
- Huang S, Li C, Armstrong EA, et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res 2013;73:824-33. [PubMed]
- Juric D, Dienstmann R, Cervantes A, et al. Safety and Pharmacokinetics/Pharmacodynamics of the First-in-Class Dual Action HER3/EGFR Antibody MEHD7945A in Locally Advanced or Metastatic Epithelial Tumors. Clin Cancer Res 2015;21:2462-70. [PubMed]
- Abel EV, Basile KJ, Kugel CH 3rd, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest 2013;123:2155-68. [PubMed]
- Girotti MR, Pedersen M, Sanchez-Laorden B, et al. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov 2013;3:158-67. [PubMed]
- Yadav V, Zhang X, Liu J, et al. Reactivation of mitogen-activated protein kinase (MAPK) pathway by FGF receptor 3 (FGFR3)/Ras mediates resistance to vemurafenib in human B-RAF V600E mutant melanoma. J Biol Chem 2012;287:28087-98. [PubMed]
- Dieci MV, Arnedos M, Andre F, et al. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer discovery 2013;3:264-79. [PubMed]
- Metzner T, Bedeir A, Held G, et al. Fibroblast growth factor receptors as therapeutic targets in human melanoma: synergism with BRAF inhibition. J Invest Dermatol 2011;131:2087-95. [PubMed]
- Kendra KL, Plummer R, Salgia R, et al. A multicenter phase I study of pazopanib in combination with paclitaxel in first-line treatment of patients with advanced solid tumors. Mol Cancer Ther 2015;14:461-9. [PubMed]
- Dayer LE, Hutchins LF, Johnson JT. Treatment of metastatic melanoma with pazopanib: A report of five patient cases. J Oncol Pharm Pract 2015;21:224-31. [PubMed]
- Gordon MS, Kluger HM, Shapiro G, et al. Activity of cabozantinib (XL184) in metastatic melanoma: Results from a phase II randomized discontinuation trial (RDT). J Clin Oncol 2012;30:abstr 8531.
- Sharfman WH, Hodi FS, Lawrence DP, et al. Results from the first-in-human (FIH) phase I study of the oral RAF inhibitor RAF265 administered daily to patients with advanced cutaneous melanoma. J Clin Oncol 2011;29:abstr 8508.
- Lesinski GB. The potential for targeting the STAT3 pathway as a novel therapy for melanoma. Future Oncol 2013;9:925-7. [PubMed]
- Carson CC, Moschos SJ, Edmiston SN, et al. IL2 Inducible T-cell Kinase, a Novel Therapeutic Target in Melanoma. Clin Cancer Res 2015;21:2167-76. [PubMed]