Current trends in the surgical management and treatment of adult glioblastoma
Introduction
Glioblastoma is a primary brain neoplasm that has been the highest funded intracranial malignancy by the NIH over the past 40 years. Treatment options have been limited and the survival rates have not changed dramatically during this time period, especially when compared to other cancers such as breast and lung cancer. Current research endeavors have not led to a cure for glioblastoma, however bench research and clinical trials have contributed to both an improved understanding of the disease progression, as well as small improvements in patient outcomes to treatment. As technology improves, scientists make strides towards a better understanding of the genomics, cellular workings, and clinical behavior of the various subtypes of glioblastoma, which look to ultimately develop a cure for this devastating disease.
According to the World Health Organization (WHO), glioblastoma is the most common primary brain neoplasm (1). It comprises 15% of all intracranial neoplasms and 60-75% of astrocytic tumors (2). The term glioblastoma multiforme (GBM) was previously synonymous with glioblastoma, however “multiforme” is currently no longer a part of the WHO classification (3). The abbreviation, GBM, is still commonly used and accepted in the literature to refer to glioblastoma. Glioblastoma is derived specifically from the astrocyte cell type, in which the cellular growth is unregulated and tumor formation occurs. Examples for the WHO grading system of astrocytomas can be seen on Table 1. Grade 1 astrocytomas are considered benign and slow growing. Surgical excision is typically considered curative, however gross total resection (GTR) can sometimes be limited by adhesions the tumor may have with surrounding eloquent anatomy. Grade 2 astrocytomas are more likely to recur or progress over time thus making surgical excision with histopathological evaluation very important. Grade 3 and 4 astrocytomas are considered high grade gliomas and GTR is desired, however like the lower grades, this may not always be possible depending on the location of the tumor. Following resection/biopsy of a WHO grade 3 and 4 astrocytoma, chemotherapy and radiation therapy is typically initiated. The discussion of purely non-operative treatments options for glioma is beyond the scope of this reading.

Full table
Presentation
Clinical presentation
The clinical presentation of a patient with a newly diagnosed GBM can vary greatly. Non-specific complaints include headaches, dizziness, nausea, lethargy, seizures, hemiparesis, visual loss, stroke-like symptoms, memory problems, or personality changes. Depending on the size and location of the tumor, the presentation can vary widely. It is also important to take into the account of surrounding edema from the tumor itself as it can cause more neurological deficit than the tumor itself. Consequently, high dose steroids in the acute setting can help improve some of the observed symptoms. Typically a loading dose of dexamethasone 10 mg IV is given followed by dexamethasone 6 mg every 6 h which can be tapered down, with the taper being surgeon specific. It is also important to pair the high dose steroid use with a gastrointestinal protectant such as a proton pump inhibitor or histamine type 2 receptor blocker.
Radiographic presentation
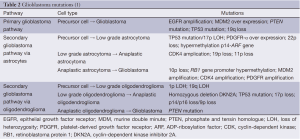
Upon presentation with neurological symptoms, the screening test of choice for the primary care physician or emergency department physician is typically a non-contrasted head CT. This quick non-invasive imaging modality can rule-in the possibility of having intracranial pathology, especially glioblastoma (4). Typical findings for glioblastoma on a contrasted head CT are a heterogeneous hyperdense ring with a hypodense core. In addition, there is typically surrounding hypodense signal tracking closely along subcortical white matter tracts indicating cerebral vasogenic edema (Figure 1) (5,6). Other common features indicating mass effect from the tumor may include sulcal effacement, midline shift, compression of the ventricles, and possibly evidence of either uncal or tentorial herniation.
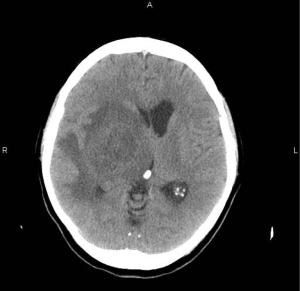
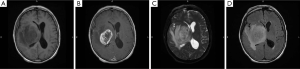
While CT provides initial data upon presentation, the imaging modality of choice for brain tumors is a contrast-enhanced MRI, including T1-/T2-weighted and FLAIR sequences. Gliomas typically appear as a contrast-enhancing mass, with a thickened rind of enhancement and a hypointense core, which corresponds to central areas of necrosis (7). The margins of the tumor may be irregular or poorly defined, with spread of the tumor along white matter tracts (Figure 2A-D) or transcallosally into the opposite hemisphere (8) (Figure 3A-D). Traditional MRI is also capable of demonstrating the degree of edema surrounding a tumor, and susceptibility imaging can show whether or not a tumor contains micro-hemorrhages. Other MRI sequences such as DWI/ADC have helped detect the cellularity of certain tumors. Some studies have claimed that ADC can be a predictor of increased tumor cellularity and perhaps indicating a higher grade of tumor such as glioblastoma from low grade astrocytomas (9,10). In certain cases, the standard MR sequences are unable to differentiate tumor tissue against non-specific tissue changes related to treatment, such as radiation necrosis. Adjunct imaging modalities, such as, MRI spectroscopy, or positron emission tomography with various radiotracers in conjunction with the standard MRI can be useful in helping with these challenges.
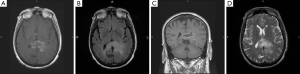
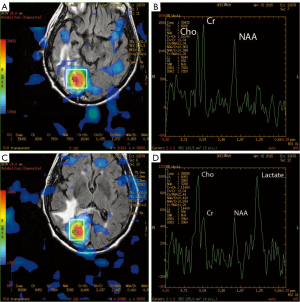
MR spectroscopy (MRS) is a method to non-invasively evaluate metabolites in tissue and relies on the resonance of these tissues at various echo times. Choline (Cho), N-acetylaspartate (NAA), lactate, lipid, and creatinine (Cr) are common cell and neuronal metabolites identified at long echo times (Figure 4). Different patterns can be associated with increased cell turnover, and specifically an increased Cho and decreased NAA peak is associated with malignancy (11,12). In addition having a lactate peak can indicate anaerobic metabolism seen commonly when the tumor outgrows its vascular supply leading to necrosis. It is also important to keep in mind that the lactate peak can also be seen in infections, however a lactate peak in conjunction with an increase in Cho and decrease in NAA is more indicative of an aggressive tumor. Another function with MRI is the dynamic susceptibility contrast (DSC), which can help evaluate blood volume within a region of interest (ROI). The ROI with increased blood volume can correlate with increased tumor vascularity and it has been shown that this can be associated with higher grade gliomas (13).
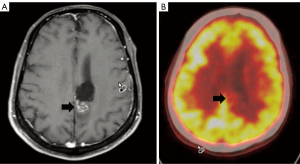
Despite being introduced as early as 1982, positron emission tomography (PET) with 2-fluoro-2-deoxy-D-glucose (FDG), has never truly gained widespread acceptance. FDG uptake into glial cells is indicative of tumor cell hypermetabolism of glucose, and FDG PET imaging returns an image signal that is superior to anatomic imaging alone for grading gliomas (Figure 5). In low-grade gliomas, glucose metabolism of tumor cells is only slightly higher than that of white matter. In contrast, the FDG uptake is significantly increased in high-grade glioma as compared to adjacent white matter and thus PET signal has a linear correlation with the glioma grade. Numerous studies have shown a correlation between pre-operative FDG PET results with actual histopathology (14) and the clinical course (15). In addition, the use of PET to delineate targets for stereotactic biopsy increases the diagnostic yield by demonstrating minute local variation in tissue metabolic profiles, and thus showing truly hypermetabolic regions within individual tumors (16,17). Additional radiotracers, such as O-(2-[18F]fluoroethyl)-L-tyrosine (F-FET), have been developed to hopefully rival FDG in efficacy (18), but as of yet no large studies have used these new tracers (19,20).
Pathology of glioblastoma
The histopathology of glioblastoma typically shows a highly cellular, poorly differentiated, pleomorphic astrocytic cells with nuclear atypia and high mitotic activity. In the background, there is usually microvascular proliferation and necrosis in certain sections (1,21,22) (Figure 6).
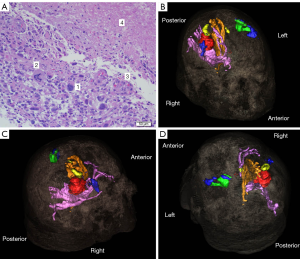
Glioblastoma arise from two pathways: (I) the primary or de novo pathway; and (II) the secondary pathway. The de novo glioblastoma come from astrocytes or precursor/stem cells that have baseline mutations that can be one or a combination of the following: loss of chromosome 10q, epithelial growth factor receptor (EGFR) amplification, murine double minute 2 (MDM2) overexpression, phosphate and tensin homologue (PTEN) gene mutation, or TP53 mutation (23). As this is just a list of the most common alterations commonly detected, this listing is by no means limiting (1) (Table 2).
Within the development of a secondary glioblastoma, there have been two alternative pathways described. The first pathway involves a WHO grade 2 astrocytoma that can either progress to a grade 3 or it can spontaneously become a grade 4 depending on the type of “hit” it receives (24). The second alternative pathway is a WHO grade 2 oligodendroglioma that becomes a grade 3 and ultimately transitions into a WHO grade 4 glioblastoma. The evolution of low grade to high grade population for secondary glioblastoma is uncommon, and is only seen in approximately 5% of total GBMs (24). Recent studies displayed that a fraction of the secondary glioblastoma may have evolved from lower grade gliomas with mutations in the isocitrate dehydrogenase 1 (IDH1) gene (25). In evaluating the IDH gene in grade 2/3 astrocytomas and oligodendrogliomas, researchers observed an increased length of survival in those with an IDH mutation versus an IDH wild-type (26). Conversely, tumors with a telomerase reverse transcriptase (TERT) mutation has demonstrated a shorter median length of survival compared to those without a mutation, 14 versus 27 months respectively (27). Research has displayed that having a TERT mutation prevents chromosomal telomerase shortening, and without the telomerase being shortened, cancer cells do not have the cell signals to undergo cell death.
Researchers at The Cancer Genome Atlas (TCGA), have made strides to determine that there are four different subtypes of glioblastoma: classical, proneural, mesenchymal, and neural (28). The classical subtype has the highest concentration of EGFR on the cell surface and the TP53 gene is not mutated. From a clinical perspective the classical subtype has the longest survival with aggressive post-operative treatment. The majority of proneural subtype are secondary GBM, with mutations in the IDH1, IDH2, and p53 genes. These patients are usually younger and with aggressive treatment they have the best prognosis. In the mesenchymal subtype, there is decreased NF1 expression with a higher expression of a family of genes related to the tumor necrosis factors. The clinical consequence of the mesenchymal subtype is an increased amount of necrosis along with associated inflammatory infiltrates. Patients typically present with worsening edema seen on radiological imaging. The fourth subtype, neural, is a proliferative type with expression of neuronal markers seen in non-cancerous neuronal cells. Patients with tumors consistent with the neural subtype typically are of older age compared to the other subtypes and with aggressive management there is some response, but not as robust compared to the classical and mesenchymal subtypes.
Operative candidate selection
Following radiographic assessment, the patient must be assessed for the appropriate surgical intervention. Establishing a diagnosis is of primary importance for any patient presenting with an intracranial mass lesion. In addition, the relationship of the tumor to eloquent cortex (i.e., primary motor cortex or primary speech centers) is also of primary importance as this relationship directly corresponds to the amount of tumor that can be resected without permanent morbidity.
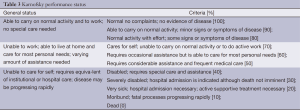
One of the factors used to determine if a patient is a good surgical candidate is the Karnosky Performance Scale (KPS) index (Table 3). This index can be used as a baseline parameter as well as a post-operative measure (29-32). Chaichana et al. found that a KPS index >90 prolonged the functional outcome of their patients (33). This would be as expected, since patients with a higher KPS index preoperatively, would likely have a higher score post-intervention. On the other hand, Marina et al. showed that patients with a low preoperative KPS index ≤50 displayed improvement by increasing their survival time and functional status (KPS index) (34). Although the literature can vary, a KPS index greater than or equal to 70 is generally the minimum for offering surgical intervention (35), but as always exception can be made in select patients with KPS index <70 which can display improved survival and quality of life following tumor cytoreduction (32,36).
Full table
Neurosurgical considerations for glioblastoma treatment
As stated above, the surgical approach must be individualized for each patient. This intervention must include the primary goals of establishing diagnosis while maintaining the patient’s pre-operative KPS. In addition, a fine balance must ensue to minimize morbidity while maximizing quality of life and extending survival.
Stereotactic needle biopsy
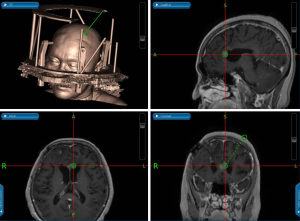
The needle biopsy is one of the least invasive ways of obtaining tissue for pathological diagnosis. This procedure is usually reserved for patients with multiple co-morbidities whom would not be able to tolerate a large cranial surgery or for those with unresectable tumors due to its location. There are a few ways a needle biopsy can be performed. The first method is to use a stereotactic system and making a small craniotomy or burr hole right over the tumor location where it comes closest to the surface and taking a biopsy. The second option is to use a stereotactic system with a frameless guided needle system to obtain tissue from a deeper location. Historical accuracy of this procedure varies between 62-95% (37). Lastly, and being the most involved, is the placement of a stereotactic frame and using Cartesian/polar coordinates (38) to obtain tumor tissue (Figure 7). This latter methods is the most accurate (39), however it also requires the most planning, equipment, computer software, and cooperation from the patient. The sensitivity and specificity that comes from this last method with the stereotactic frame needle biopsy is 90.7% and 100% respectively (40).
Awake craniotomy
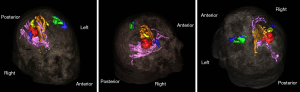
Awake craniotomy is performed when a glioma is located near eloquent cortex in areas that includes, but not limited to, the precentral gyrus (motor strip), corticospinal tracts, Broca’s speech area, Wernicke’s speech area, and the brainstem (Figure 8). During an awake craniotomy the patient is sedated and is awoken once cortical stimulation or testing is about to begin. During this time, a neurologist will assist the neurosurgeon in stimulating the cortex and mapping the brain around the tumor to locate areas that are eloquent. This allows the neurosurgeon to determine which areas are outside the eloquent cortex and safe for resection. This technique can be of value for lesions that are on the surface to determine which portions of the tumor are resectable as well as deep seated tumors to determine a safe trajectory to the lesion. Once the mapping is completed, the patient is sedated for the remainder of the case. There are multiple limitations in performing an awake craniotomy (41). Patients cannot be claustrophobic, have psychiatric issues such as anxiety, or neurocognitive issues such as dementia or mental retardation.
Standard craniotomy approach
The majority of glioma surgery entails a standard craniotomy approach with the patient under general anesthesia. Prior to intra-operative navigation, craniometrics were used and larger incisions/craniotomies were created to have adequate exposure (42). Currently with the use of intra-operative navigation systems, the surgeon can precisely determine the tumor location and plan the incision and craniotomy accordingly (43). This helps decrease operative time, limit the size of the incision and craniotomy, and it can also help determine the extent of resection (EOR) in relation to nearby critical structures.
Extent of resection (EOR)
Glioblastoma is the most common primary malignant brain tumor in adults. Given its poor prognosis, treatment and determination of EOR to enhance overall survival continues to be a greatly researched topic. At this time, the mainstay of treatment for newly diagnosed glioblastoma is microsurgical resection with additional radiation therapy and chemotherapy. Notwithstanding advances in surgical procedure and technique, this multimodal approach only results in a median overall survival of 12-15 months (44,45). Given that no other modalities have appeared to supplant this surgical approach, it still remains the first line therapy. Interestingly, the most compelling evidence exists for low-grade gliomas, where volumetric analyses have shown in both insular and hemispheric lesions, greater EOR is associated with a survival benefit (46), specifically improved overall survival, progression-free survival, and malignant progression-free survival. In contrast, evidence for high-grade gliomas has been more inconsistent.
The role of surgery, in specific the EOR, has historically been a controversial topic. Lacroix and colleagues were the first to demonstrate that not only resection, but EOR is associated with longer survival time in GBM patients. Their retrospective analysis of 416 patients with newly diagnosed and recurrent GBM concluded that a ≥98% EOR is necessary to improve survival significantly. Furthermore, they showed a hazard ratio of 1.4 for patients who underwent subtotal resection (STR) versus GTR at initial presentation, which proved to be an independent predictor of survival (29). However, this study was severely hampered by several design deficiencies, specifically combining both newly diagnosed and recurrent GBMs into the same study despite considerable difference in their biological features and outcome. Nevertheless, due to a lack of a more comprehensive analysis, this EOR study served as a critical study of reference in justifying the “all-or-none” approach in surgical management of GBM (46). Since this publication multiple other groups have corroborated these results, demonstrating increased overall survival associated with greater EOR in GBM patients (47), including the elderly who are thought to have poorer outcomes regardless of intervention (32,48).
In one of the largest studies to date that included 500 newly diagnosed glioblastoma patients, Sanai and colleagues from University of California, San Francisco, demonstrated that an EOR as low as 78% is associated with improved overall survival. Step-wise improvement in overall survival was shown beyond these margins and even at the highest levels of resection, all together suggesting the utility of both sub-total and gross-total resection in the treatment of newly diagnosed glioblastomas (46). In trying to achieve GTR, Orringer and colleagues performed a volumetric analysis of 46 patients and found that patients with an EOR greater than 90% was associated with a significantly greater 1-year survival (76.5%) (49).
The ultimate goal of resection of recurrent glioblastomas is to establish a diagnosis and relieve the mass effect. Beyond this, the clinical value of resection continues to be controversial. Recent evidence suggests that both gross and sub-total resection can benefit patients requiring re-resection at tumor recurrence. However, there are significant differences in the neurological morbidity seen in those patients with more extensive resection, specifically during the early post-operative period. Oppenlander et al. reported that at 7 days after surgery, a deterioration in the NIHSS score by 1 point or more was observed in 39.1% of patient with EOR ≥80% compared with 16.7% for those with EOR <80% (P=0.0049). Furthermore, greater EOR is associated with a higher percentage of permanent deficits, with a deterioration of the NIHSS score by at least 1 point 30 days post-operatively in 31.3% of patients with ≥95% EOR, compared with 18% for those with EOR <95%. Distinguishing between gross-total resection and sub-total resection provides a foundation for clinical decision making for the neurosurgical oncologist (50). However, the arbitrary assignment of a 95% EOR threshold (45) separating these two groups simplifies the intraoperative challenges the neurosurgeon has to deal with. Ultimately, attaining EOR beyond the 80% threshold, while preserving functional pathways to maximize quality of life, should be the goal of the neurosurgeon treating patients with recurrent glioblastoma (50).
In regards to the impact of EOR at initial and repeat craniotomy, specifically regarding the cumulative effect on overall survival, Bloch et al. demonstrated evidence that if GTR is achieved at initial resection, the EOR at repeat craniotomy does not affect overall survival. However, if the initial resection is subtotal, a subsequent GTR thereafter significantly improves overall survival, suggesting those patients with an initial STR, would likely benefit from a subsequent GTR (47).
While EOR has long been considered as the most accurate and significant predictor of outcome, recent studies suggest other measurements may be of greater value. Chaichana et al. retrospectively evaluated 259 patients who underwent primary GBM surgery from 2007 to 2011 and performed a volumetric analysis showing for the first time that the minimum EOR of 70% and the maximal residual volume (RV) of 5 cm3 showed statistical significance for improved survival with decrease of recurrence. This can be compared to prior studies of having the minimal EOR to be 98% and 78% established by Lacroix and Sanai, respectively. The trend of having a smaller percentage of EOR could be attributed to the fact that since the Stupp trial was published in 2005, these studies have now adopted temozolomide as standard of care for all patients. By having modern adjuvant therapies, it could be postulated that these therapies are more effective at treating larger residual tumor volume (RTV), and thus less total resection may be required. With regards to postoperative RTV, this study showed that surgery itself may have an impact on survival, regardless of preoperative tumor volume. It makes intuitive sense that the higher the tumor burden, the worse the outcome as there is likely to be a higher number of residual tumor cells that are resistant to adjuvant therapy (51).
In a more recent retrospective review by Grabowski et al., they evaluated 128 patients who underwent primary resection of GBM, volumetric analysis of pre- and post-operative MR images was performed and the results demonstrated that contrast enhancing RTV (CE-RTV), T2 FLAIR residual volume (T2/F-RV), and EOR were predictive of survival. Most importantly, the study demonstrated that CE-RTV was a stronger predictor of survival than EOR or T2/F-RV, with significant survival benefit noted for residual volumes of less than 2 cm3 of contrast-enhancing disease (52), while Chaichana et al. reported a RTV of 5 cm3 to have significant in overall survival (51). From these studies, it appears that CE-RTV is a more important predictor of outcome than the EOR, and that the residual enhancing tumor burden, prior to the start of medical therapy, is an important prognostic factor. However, EOR does not provide a true indication of residual microscopic disease burden, and in fact the biological impact of EOR may be more dependent on the preoperative tumor volume. A near total resection of a large tumor may leave behind more residual tumor than a STR of small tumor, and yet the buttressed relationship of EOR and outcome would suggest that a greater completed resection is associated with improved outcome, regardless of residual burden of disease. At this point, further studies are still needed to clarify the effect of CE-RTV on overall survival after GBM resection (52).
Surgical adjuncts
Greater emphasis on the importance of achieving maximal resection has led to the development of new methods at maximizing the safety, precision, and extensiveness of resections including neuronavigation, functional mapping, intraoperative fluorescent dyes, and intraoperative MRI (iMRI) (47). Prior to the initiation of adjuvant treatment, the primary therapeutic goal should be maximal safe surgical resection with preservation of neurological function. To date there are several surgical resection techniques, including functional MRI (fMRI), iMRI, and 5-aminolevulinic acid (5-ALA).
Diffusion tensor imaging (DTI) tractography is a form of diffusion-weighted MR imaging that can provide images of important white matter tracts within the central nervous system (CNS) by assessing physiological water directionality and motion (53). In contrast, conventional MRI only provides anatomical information, providing little information about CNS connectivity. However, through the use of DTI, the neurosurgeon is able to identify clinically relevant white matter tracts and can devise an improved and appropriate surgical approach for glioblastoma resection in or near eloquent areas (54).
The most obvious challenge associated with DTI is brain shift. While preoperative DTI imaging has been shown to have high fidelity, inward and outward shifts of brain matter occur once the dura mater is opened. To correct and account for possible shifts, many centers use ultrasound, iMRI, or direct stimulation to verify anatomical locations intraoperatively. Further, gliomas and other lesions can distort CNS fiber tracts by invasion or vasogenic edema, thus impeding the motion and normal directionality of water molecules in the brain and lower the anisotropy value. This intrinsic problem with DTI supports a more multimodal approach to glioblastoma resection with fMRI (54). In combination, these imaging methods allow for overlay of functional and anatomical spatial relationships. Gonzalez-Darder and colleagues utilized this approach in the resection of gliomas in eloquent motor areas and found that in all 17 cases, identification of the central sulcus and precentral gyrus was easily apparent versus 11 of the 17 when traditional intraoperative visual inspection was employed (55). Further, Ius et al. investigated the role of EOR in overall survival in patients with low-grade gliomas and found that the EOR was significantly greater in those patients who had undergone DTI- and fMRI neuronavigation as compared to subcortical stimulation alone (90% vs. 77%) (56).
The use of iMRI has increased over the past decade, with the specific aim of achieving a greater EOR. iMRI allows for visualization of contrast-enhancing tumor underneath and on the surface of the resection cavity, and more importantly it allows the ability to reassess neuronavigation during surgery thus, providing the opportunity to selectively continue tumor resection (57). While conventional neuronavigation is based upon preoperative imaging, as seen with pre-operative DTI, the anatomy can alter during surgery secondary to tumor resection and loss of CSF resulting in brain shift. This can render conventional neuronavigation inaccurate after opening of the dura, and ultimately causing tumor remnants to be missed. iMRI can be utilized to identify these remnants by restoring the accuracy of neuronavigation. However, the process can be quite time consuming during acquisition of these static images, increasing operating room (OR) time by up to 1 h when compared to conventional surgery and is also associated with higher maintenance costs (58,59).
The literature suggests that there’s a benefit in around 30-40% of all cases with regards to EOR of contrast-enhancing lesions with iMRI, when comparing intraoperative and postoperative MR scans. Only a few studies have closely analyzed the efficacy and benefit of iMRI-guided versus conventional resection in terms of EOR and survival, showing a benefit in both cases (59-61). Among these studies was a randomized controlled trial which showed an increased rate of achieved GTR using iMRI (96% achieved GTR with iMRI versus 68% without iMRI) (59). In a more recent randomized, triple-blind, parallel controlled trial, the effect of iMRI-guided glioma resection on surgical efficiency, morbidity, overall survival, and progression-free survival of gliomas was evaluated, and suggested a significantly higher rate of GTR in the iMRI group (86.36%) than in the control or conventional neuronavigation group (53.49%) (62).
However, due to the high costs of iMRI, as well as the desire to continue to improve patient treatment and survival, there has been increased interest in comparing this method to other cheaper and more conventional resection modalities like 5-ALA.
Typically 5-ALA is given orally preoperatively and is used as a technical adjunct by accumulating in glial tumor cells and provides a fluorescence that can be detected using the intraoperative microscope with appropriate lens filters. One study has shown that using 5-ALA has increased the likelihood of achieving a GTR compared to white light only (65% vs. 37%) (63), while others have shown a greater extent/volume of resection when compared to contrasted scans (64). Through biochemical reactions that result in increased uptake in tumor cells, 5-ALA allows for excellent resection margin control (65). It is an advantage to use 5-ALA since it provides the capability to identify fluorescent tumor tissue in real-time within the resection cavity. In addition, aside from the cost of the microscope attachment, 5-ALA itself is purchased at a negligible cost. However, the efficacy of 5-ALA is entirely dependent on direct visualization of the fluorescent tissue areas. The use of 5-ALA alone can lead to the erroneous impression of complete tumor resection, especially in cases of poor direct visualization or the presence of a thin intervening margin of non-pathological tissue (66). Furthermore, the use of 5-ALA without functional data in the setting of tumor resection, increases the risk of post-operative neurological deficits secondary to resection of eloquent brain areas (57). A recent single institutional prospective study sought to evaluate whether 5-ALA fluorescence provides an additional benefit in detection of invasive tumor compared with iMRI. While sensitivity for tumor detection was significantly higher in 5-ALA (0.85) than in iMRI (0.41), specificity was significantly lower in 5-ALA (0.43) than in iMRI (0.70). For detection of pathological tissues, 5-ALA significantly exceeded iMRI in specificity (0.8 vs. 0.6) and sensitivity (0.91 vs. 0.66), suggesting a combined approach of 5-ALA in addition to iMRI might be beneficial to maximize EOR in high grade gliomas (67).
A key prognostic factor in neurosurgical oncology is the EOR. While image guided surgery is often used to identify and map the resection margin, it is not clear whether these, sometimes very expensive, tools should be the established standard of care for all glioma patients. A recent Cochrane study found low to very low quality evidence that image-guided surgery using iMRI, 5-ALA or DTI-neuronavigation increases the proportion of patient with complete tumor resection on post-operative MRI. Moreover, theoretical concerns that maximizing the EOR would lead to more frequent adverse side effects was poorly reported in the included studies, as were the effects of image guidance on survival and quality of life (68). Despite these findings, these modalities are thus far the most efficacious methods available for accurate and precise tumor resection.
Prognosis and what to expect after surgery
Despite advances in treatment of glioblastoma, the prognosis is poor compared to other intracranial neoplasms. The median survival with current therapies ranges from 9 to 22 months (69). The length of survival is multifactorial as shown in the literature (30) which has included, age, EOR, subtypes of glioblastoma, response to chemotherapy and radiation, methylation status of O-6-methylguanine-DNA methyltransferase (MGMT), KPS score etc. (31,70). Although there have been trials using different chemotherapeutics, different radiation modalities, immunotherapy, and gene therapy using viral vectors, these studies are still under investigation.
The specific details of chemotherapy and radiation therapy are beyond the scope of this paper. Suffice to say, the current therapy using temozolomide (Temodar®) and conformal radiation is currently accepted as first line standard of care therapy among neuro-oncologists (71). Temozolomide is a alkylating agent that works by inserting a methyl group into the guanine DNA base, thus damaging the DNA and triggering cell death (71). As mentioned above, this chemotherapy drug can sometime be mitigated by the MGMT methylation status of a patient. There is a protein O6-alkyguanine DNA alkytransferase (AGT) that is encoded by the MGMT gene, and it is this AGT protein that has the ability to repair DNA by removing the methyl group inserted by temozolomide. In the event that a patient does not have the MGMT gene methylated, the AGT protein is created and it can decrease the efficacy of temozolomide. However if the MGMT gene is methylated (thus silencing the gene), the AGT protein is not translated and the tumor cells are more susceptible to temozolomide resulting in a greater efficacy and response to treatment.
Despite maximal surgical resection following by adjuvant chemoradiation the median time to tumor recurrence is 8 months (51). One of the primary theories on the reason for such short of a response is the rapid growth of residual cancer stem cells and the ability of this cells to modify their genetic signature (72). From a treatment perspective, many times the benefit for re-resection is for cytoreduction (50) prior to starting a second line chemotherapy agent bevacizumab (Avastin®), known as a vascular endothelial growth factor inhibitor, thus preventing angiogenesis. Currently bevacizumab is the only FDA approved treatment for recurrent glioblastoma. There have been clinical studies for the treatment of recurrent glioblastoma such as re-irradiation or stereotactic radiosurgery, however no studies have shown statistical efficacy when compared to bevacizumab (73).
In terms of active cancer research in treating glioblastoma, current research is battling this disease from multiple fronts: tyrosine kinase inhibitors, integrin inhibitors, gene therapy, and mammalian target of rapamycin (mTOR), just to name a few. Neoadjuvant chemotherapy, such as the phase II study using bevacizumab + chemoradiotherapy (temozolomide) (74), is being explored in hopes to make newly diagnosed glioblastoma more amendable to GTR during surgery. Gilbert et al. published in New England Journal of Medicine their randomized trial of bevacizumab in newly diagnosed glioblastoma. Although their results did not show improvement in overall survival, it did show a trend towards prolonged progression-free survival (75). The result of this study was monitored with serial MRIs, which can be misleading since it is known that bevacizumab can make gliomas “disappear” radiologically due to its mechanism of action. Others groups have explored other adjuvant agents used in combination with bevacizumab and temozolomide such as irinotecan, an inhibitor of the DNA topoisomerase 1 typically used in colon cancer, which also has not shown statistical improvement in survival benefit (76). Other phase I/II studies using erlotinib and temsirolimus (77) sorafenib with temsirolimus (78) for recurrent glioblastoma have proven the safety of their usage, but their efficacy is still to be determined at this time.
The treating team of neurosurgeons, neuro-oncologists, radiation-oncologists, and primary care providers must discuss each individual patient and determine the role of surgical re-resection with regard to second line chemotherapeutics while taking into account morbidity and quality of life. Despite the short median length of survival, there exist case reports of long-term survival with glioblastoma (79). However, it is thought that some of these patients may have either been “misdiagnosed” or perhaps the tumor was of secondary glioblastoma heritage originating from the oligodendrocyte lineage (80). There is consideration for re-resection for the purpose of cytoreduction prior to starting a new chemotherapeutic. This approach, however, is controversial and must be considered on an individual basis.
Conclusions
Glioblastoma is disease without a cure despite extensive research involving surgical resection and (neo)adjuvant therapy. However, small strides have been made and maximal surgical resection is shown as a key component of treatment. Numerous surgical adjuncts such as DTI, fMRI, iMRI and 5-ALA have proven efficacious in this regard. The key to a cure still relies on post-operative treatment and we will need to rely on further basic science research and clinical trials to lead us to that end.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Louis DN, International Agency for Research on Cancer, World Health Organization. WHO classification of tumours of the central nervous system. 4th ed. Lyon: International Agency for Research on Cancer, 2007.
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 2014;16 Suppl 4:iv1-63. [PubMed]
- Ellor SV, Pagano-Young TA, Avgeropoulos NG. Glioblastoma: background, standard treatment paradigms, and supportive care considerations. J Law Med Ethics 2014;42:171-82. [PubMed]
- Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med 1993;11:253-8. [PubMed]
- Witwer BP, Moftakhar R, Hasan KM, et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J Neurosurg 2002;97:568-75. [PubMed]
- Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology 1989;170:211-7. [PubMed]
- Osborn AG, Salzman KL, Katzman G, et al. Diagnostic Imaging: Brain. 1st ed. Salt Lake City: Amirsys, 2004.
- Bourekas EC, Varakis K, Bruns D, et al. Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol 2002;179:251-7. [PubMed]
- Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001;22:1081-8. [PubMed]
- Server A, Kulle B, Maehlen J, et al. Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol 2009;50:682-9. [PubMed]
- Deviers A, Ken S, Filleron T, et al. Evaluation of the lactate-to-N-acetyl-aspartate ratio defined with magnetic resonance spectroscopic imaging before radiation therapy as a new predictive marker of the site of relapse in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys 2014;90:385-93. [PubMed]
- Caivano R, Lotumolo A, Rabasco P, et al. 3 Tesla magnetic resonance spectroscopy: cerebral gliomas vs. metastatic brain tumors. Our experience and review of the literature. Int J Neurosci 2013;123:537-43. [PubMed]
- Hipp SJ, Steffen-Smith E, Hammoud D, et al. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol 2011;13:904-9. [PubMed]
- Massager N, David P, Goldman S, et al. Combined magnetic resonance imaging- and positron emission tomography-guided stereotactic biopsy in brainstem mass lesions: diagnostic yield in a series of 30 patients. J Neurosurg 2000;93:951-7. [PubMed]
- Padma MV, Said S, Jacobs M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol 2003;64:227-37. [PubMed]
- Levivier M, Goldman S, Pirotte B, et al. Diagnostic yield of stereotactic brain biopsy guided by positron emission tomography with [18F]fluorodeoxyglucose. J Neurosurg 1995;82:445-52. [PubMed]
- Pirotte B, Goldman S, Dewitte O, et al. Integrated positron emission tomography and magnetic resonance imaging-guided resection of brain tumors: a report of 103 consecutive procedures. J Neurosurg 2006;104:238-53. [PubMed]
- Pauleit D, Stoffels G, Bachofner A, et al. Comparison of (18)F-FET and (18)F-FDG PET in brain tumors. Nucl Med Biol 2009;36:779-87. [PubMed]
- Wester HJ, Herz M, Weber W, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med 1999;40:205-12. [PubMed]
- Wang HE, Wu SY, Chang CW, et al. Evaluation of F-18-labeled amino acid derivatives and [18F]FDG as PET probes in a brain tumor-bearing animal model. Nucl Med Biol 2005;32:367-75. [PubMed]
- Reifenberger G, Collins VP. Pathology and molecular genetics of astrocytic gliomas. J Mol Med (Berl) 2004;82:656-70. [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [PubMed]
- Renault IZ, Golgher D. Molecular genetics of glioblastomas: defining subtypes and understanding the biology. Neuroimaging Clin N Am 2015;25:97-103. [PubMed]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol 2007;170:1445-53. [PubMed]
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008;321:1807-12. [PubMed]
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765-73. [PubMed]
- Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A 2013;110:6021-6. [PubMed]
- Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010;17:98-110. [PubMed]
- Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190-8. [PubMed]
- Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 2003;99:467-73. [PubMed]
- Scott JN, Rewcastle NB, Brasher PM, et al. Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci 1998;25:197-201. [PubMed]
- Ewelt C, Goeppert M, Rapp M, et al. Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neurooncol 2011;103:611-8. [PubMed]
- Chaichana KL, Halthore AN, Parker SL, et al. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg 2011;114:604-12. [PubMed]
- Marina O, Suh JH, Reddy CA, et al. Treatment outcomes for patients with glioblastoma multiforme and a low Karnofsky Performance Scale score on presentation to a tertiary care institution. Clinical article. J Neurosurg 2011;115:220-9. [PubMed]
- Chambless LB, Kistka HM, Parker SL, et al. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol 2015;121:359-64. [PubMed]
- Watts C, Price SJ, Santarius T. Current concepts in the surgical management of glioma patients. Clin Oncol (R Coll Radiol) 2014;26:385-94. [PubMed]
- Jackson RJ, Fuller GN, Abi-Said D, et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 2001;3:193-200. [PubMed]
- Couldwell WT, Apuzzo ML. Initial experience related to the use of the Cosman-Roberts-Wells stereotactic instrument. Technical note. J Neurosurg 1990;72:145-8. [PubMed]
- Woodworth G, McGirt MJ, Samdani A, et al. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol Res 2005;27:358-62. [PubMed]
- Seliem RM, Assaad MW, Gorombey SJ, et al. Fine-needle aspiration biopsy of the central nervous system performed freehand under computed tomography guidance without stereotactic instrumentation. Cancer 2003;99:277-84. [PubMed]
- Meyer FB, Bates LM, Goerss SJ, et al. Awake craniotomy for aggressive resection of primary gliomas located in eloquent brain. Mayo Clin Proc 2001;76:677-87. [PubMed]
- Ciric I, Ammirati M, Vick N, et al. Supratentorial gliomas: surgical considerations and immediate postoperative results. Gross total resection versus partial resection. Neurosurgery 1987;21:21-6. [PubMed]
- Romano A, D'Andrea G, Minniti G, et al. Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur Radiol 2009;19:2798-808. [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [PubMed]
- Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2011;115:3-8. [PubMed]
- Bloch O, Han SJ, Cha S, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg 2012;117:1032-8. [PubMed]
- Gállego Pérez-Larraya J, Delattre JY. Management of elderly patients with gliomas. Oncologist 2014;19:1258-67. [PubMed]
- Orringer D, Lau D, Khatri S, et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 2012;117:851-9. [PubMed]
- Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 2014;120:846-53. [PubMed]
- Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol 2014;16:113-22. [PubMed]
- Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg 2014;121:1115-23. [PubMed]
- Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 2004;25:356-69. [PubMed]
- Abdullah KG, Lubelski D, Nucifora PG, et al. Use of diffusion tensor imaging in glioma resection. Neurosurg Focus 2013;34:E1. [PubMed]
- González-Darder JM, González-López P, Talamantes F, et al. Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus 2010;28:E5. [PubMed]
- Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg 2012;117:1039-52. [PubMed]
- Eyüpoglu IY, Hore N, Savaskan NE, et al. Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS One 2012;7:e44885. [PubMed]
- Roder C, Bisdas S, Ebner FH, et al. Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 2014;40:297-304. [PubMed]
- Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 2011;12:997-1003. [PubMed]
- Senft C, Franz K, Blasel S, et al. Influence of iMRI-guidance on the extent of resection and survival of patients with glioblastoma multiforme. Technol Cancer Res Treat 2010;9:339-46. [PubMed]
- Mehdorn HM, Schwartz F, Dawirs S, et al. High-field iMRI in glioblastoma surgery: improvement of resection radicality and survival for the patient? Acta Neurochir Suppl 2011;109:103-6. [PubMed]
- Wu JS, Gong X, Song YY, et al. 3.0-T intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 2014;61 Suppl 1:145-54. [PubMed]
- Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392-401. [PubMed]
- Schucht P, Knittel S, Slotboom J, et al. 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien) 2014;156:305-12; discussion 312. [PubMed]
- Stepp H, Beck T, Pongratz T, et al. ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol 2007;26:157-64. [PubMed]
- Ritz R, Feigl GC, Schuhmann MU, et al. Use of 5-ALA fluorescence guided endoscopic biopsy of a deep-seated primary malignant brain tumor. J Neurosurg 2011;114:1410-3. [PubMed]
- Coburger J, Engelke J, Scheuerle A, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 2014;36:E3. [PubMed]
- Barone DG, Lawrie TA, Hart MG. Image guided surgery for the resection of brain tumours. Cochrane Database Syst Rev 2014;1:CD009685. [PubMed]
- Johnson DR, O'Neill BP. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 2012;107:359-64. [PubMed]
- Walid MS. Prognostic factors for long-term survival after glioblastoma. Perm J 2008;12:45-8. [PubMed]
- Taylor JW, Schiff D. Treatment considerations for MGMT-unmethylated glioblastoma. Curr Neurol Neurosci Rep 2015;15:507. [PubMed]
- Johnson BE, Mazor T, Hong C, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014;343:189-93. [PubMed]
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-40. [PubMed]
- van Linde ME, Verhoeff JJ, Richel DJ, et al. Bevacizumab in combination with radiotherapy and temozolomide for patients with newly diagnosed glioblastoma multiforme. Oncologist 2015;20:107-8. [PubMed]
- Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370:699-708. [PubMed]
- Hofland KF, Hansen S, Sorensen M, et al. Neoadjuvant bevacizumab and irinotecan versus bevacizumab and temozolomide followed by concomitant chemoradiotherapy in newly diagnosed glioblastoma multiforme: A randomized phase II study. Acta Oncol 2014;53:939-44. [PubMed]
- Wen PY, Chang SM, Lamborn KR, et al. Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol 2014;16:567-78. [PubMed]
- Lee EQ, Kuhn J, Lamborn KR, et al. Phase I/II study of sorafenib in combination with temsirolimus for recurrent glioblastoma or gliosarcoma: North American Brain Tumor Consortium study 05-02. Neuro Oncol 2012;14:1511-8. [PubMed]
- Hottinger AF, Yoon H, DeAngelis LM, et al. Neurological outcome of long-term glioblastoma survivors. J Neurooncol 2009;95:301-5. [PubMed]
- Reifenberger G, Weber RG, Riehmer V, et al. Molecular characterization of long-term survivors of glioblastoma using genome- and transcriptome-wide profiling. Int J Cancer 2014;135:1822-31. [PubMed]