Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is a chronic, multifactorial neurodegenerative disorder in people over the age of 65 (1), which creates a huge burden to affected individuals, their families, and society (2). Pathological Characters of AD including: extracellular deposition of β-amyliod (Aβ) and intracellular neurofibrillary tangles (3,4), which is believed to play an important role in the pathogenesis of this disease. Aβ deposition and tau protein cause loss of synaptic function, mitochondrial damage, activation of microglia, and the final neuronal death (5). However, it is becoming increasingly evident that neuroinflammation cascades mediated by primed microglia cells also contribute to AD pathogenesis (6). Recently, a wealth of information linking the pro-inflammatory cytokines [such as IL-1, IL-6, and tumor necrosis factor-α (TNF-α)] released from microglia has received considerable attention for its role in AD.
As the most common immune cells in the central nervous system (CNS), microglia has long been a hotspot in AD due to their dramatic responses to the pathophysiology of the disease. Microglia activation have dual effects on AD progression: one side, activation of microglia leads to reducing Aβ accumulation by increasing its phagocytosis, clearance and degradation, which prevents the formation of amyloid plaques in the brain. On the other side, prolonged microglia activation leads to the release of pro-inflammatory cytokines, which initiates a pro-inflammatory cascade and subsequently contributes to neuronal damage and losses (7-9). In this article, we review the recent findings of pro-inflammatory cytokines released from microglia, speculate its possible role in AD progression, and present the recent advances and challenges in targeting pro-inflammatory cytokines for AD therapy.
The effects of microglia, detrimental or beneficial?
As the resident immune cell of the CNS, microglia plays a nuanced and complex role in the progression of AD (10). Recently, microglia became more important than ever before in demonstrating strong genetic implications for microglia molecules and the immune system, especially when genome-wide association study (GWAS) have identified variants of several immune genes are risk factors for late onset Alzheimer’s disease (LOAD) (11,12). These genes include CD33 (11-13), TREM2 (14,15) and the HLA-DRB4-DRB1 region (16), etc. Under physiological conditions, microglia are active to maintenance of homoeostasis, neuroprotection and neuro repair by release growth factors such as brain-derived neurotrophic factor (BDNF) and transforming growth factor (TGF) β with ramified morphology. Under pathological conditions, such as altered neuronal function, infection, injury, ischemia, and inflammation, microglia become activated, proliferate and change from a ramified to an amoeboid, macrophage-like morphology.
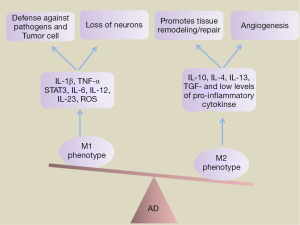
Depending on their activation status and the encountered pathologic events, microglia are able to exert a variety of effecter functions, which may be either neurotoxic or neuroprotective (17). Microglia activated by lipopolysaccharide (LPS), IFN-γ or TNF-α is considered to be ‘‘M1’’ (‘‘classically activated’’) form of microglial (18,19). M1-skewed microglial activation plays a vital role in the defense against pathogens and tumor cell by production of proinflammatory cytokines, such as IL-1β, TNF-α, STAT3, IL-6, IL-12, IL-23 and free radicals such as reactive oxygen species (ROS). Besides, it also associated with the loss of neurons. Conversely, the alternative M2 anti-inflammatory phenotype promotes tissue remodeling/repair and angiogenesis through release high levels of anti-inflammatory cytokines such as IL-10, IL-4, IL-13, and TGF-β, and low levels of pro-inflammatory cytokines (20) (Figure 1). For AD mouse models, there have been reported increase in both M1 (such as IL-1β, TNF-a, iNOS, and IL-6) and M2 (notably YM1, Arg-1, Mrc and IL-10) markers compared to age-matched wild-type controls. During the course of disease, microglia undergoes a switch from a neuroprotective to a more classically activated phenotype. In consistent with this finding, it has been proved that the later stages of AD microglia undergo a switch from a neuroprotective M2 to a more classically activated phenotype (Figure 2). NALP3 inflammasome, which mediates IL-1β production, is one of the important players driving the innate immune response towards Aβ and determining the cytokine milieu in the brain. Recently, Heneka et al. revealed that inhibition of NLRP3 can induce microglial phagocytosis and to an M2 phenotype (21). These findings are often viewed as evidence that microglia activation adapt to different stimulatory contexts and pass through a sequence of reactive profiles. The delicate balance between the pro-inflammatory and anti-inflammatory or neurotoxic and neuroprotective determines the role of microglia in a disease or condition.
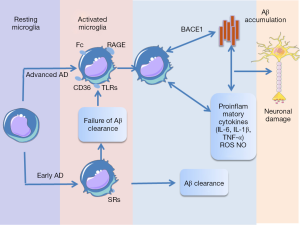
In AD, the microglia has the capacity to respond to various stimuli, including amyloid peptides, their precursor protein (APP), and neurofibrillary tangles (22). In the early stages of AD, microglial activation can promote Aβ clearance via microglia’s scavenger receptors (SRs) (23), and hinder the AD progression. The persistent microglial activation stimulated by Aβ via the receptor for CD36 (24), Fc receptors, toll-like receptors (TLRs) (25), and complement receptors advanced glycation end products (RAGE) (26), can increase Aβ production and decrease Aβ clearance, ultimately cause neuronal damage. Inhibiting Aβ-induced microglial activation can relieve the inflammatory cytokines production (27), lower Aβ deposition (28) and also ameliorate behavioral damage in vivo (29). These evidences suggest that Aβ indirectly contribute to activation of inflammatory systems, then lead to progression of AD. In 2008, Sastre et al. found that inflammation increase Aβ generation via β-secretase (BACE1), the main enzyme responsible for Aβ generation (30), therefore creating a feed-forward loop (Figure 2). What’s more, these molecular mediators of neuroinflammation have also been linked with tau-mediated neurodegeneration (31).
In parallel with their negative effects, microglia also play a beneficial role in reducing Aβ accumulation by increasing its phagocytosis, clearance and degradation in early stages of AD (32). Recently, Parkhurst et al. also found that microglia promote learning-related synapse formation through BDNF signaling in learning and memory with CX3CR1CreER mice (33). However, environmental stimulus, signal specificity, and intercellular influence decide whether the activated microglia plays a detrimental or beneficial role on the adult brain. Future studies have the task to investigate this double-edged sword.
Dysregulation of pro-inflammatory cytokine in AD
Dysregulation of pro-inflammatory cytokines in the brain
Experimental and clinical evidence have demonstrated the increased synthesis of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, IL-6, IL-18, and the upregulation of their cognate receptors in the AD brain (34-37). However, the interactions between pro-inflammatory cytokines and senile plaques—a cardinal feature of AD have been reported. Chronic deposition of Aβ in brain drives cerebral neuroinflammation by activating microglia, which is reported to be a major source of pro-inflammatory cytokines in AD (38). Aβ binding to the microglial cell surface induces pro-inflammatory gene expression and results in the elevation of pro-inflammatory cytokine such as TNF-α, IL-1β, IL-6, IL-18, which lead to tau hyperphosphorylation and neuronal loss (39). Additionally, studies showed that the levels of transcripts for a number of pro-inflammatory markers such as TNF and IL-1β were elevated in AD, specifically in response to tau (40,41). As indicated above, chronic inflammation could be the consequence of AD pathology that further exacerbates the deleterious effects exerted by Aβ and tau. However, this interpretation have been questioned and new data assumed that neuroinflammation can also be a cause of AD for increase in Aβ and tau phosphorylation in the brain (42). In support of this, previous study has revealed that transgenic mice inducing neuroinflammation by injecting LPS triggered intracellular Aβ deposition (43,44) and tau phosphorylation (45) in the brain. Yet, intriguingly, previous studies reported that synergistic effects may also occur between pro-inflammatory cytokines and Aβ, such as, IFN-γ synergize with Aβ causing the release of TNF-α and reactive nitrogen species which is toxic to neurons.
Early expression of pro-inflammatory cytokines in the AD brain by non-neuronal cells, including endothelial cells, likely plays an important role in the development of disease. AD brain microvessels release significantly higher levels of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6 than microvessels in age-matched controls (46). The cerebral microvasculature participates in a destructive cycle of events where inflammation precedes Aβ deposition and Aβ in turn promotes release of inflammatory mediators. In this aspect, exposure of brain endothelial cells to Aβ arises a series of proinflammatory responses. This is supported by the finding that exposure of cultured human brain endothelial cells to Aβ1-40 up-regulate expression of inflammatory genes IL-1β and IL-6, which is confirmed by quantitative RT-PCR analysis (47). These results suggest that the cerebral microcirculation contributes proinflammatory cytokines to the milieu of the AD brain and may be involved in the pathogenesis of neuronal injury and death in this disorder.
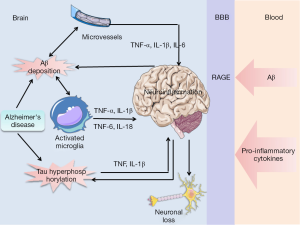
In addition, several studies (48,49) suggest that sustained inflammation from the periphery can cause pro-inflammatory cytokines in the CNS by crossing the blood-brain barrier (BBB) and can contribute to cognitive decline in AD patients. Indeed, Capuron and Miller (50) recently reviewed the pathways for the transport of pro-inflammatory cytokines to brain from systemic circulation. In addition, Aβ could also across the BBB from the periphery into brain, which is mediated by RAGE (51). And the Aβ binding to RAGE on microglia permitted the microglia to undergo the sustained activation and inflammatory response, resulting in increased proinflammatory cytokines (Figure 3). In America, studies have shown that Aβ associated BBB leakage could be present in patients with cerebral amyloid angiopathy, which affects most patient with AD (52). Based on these findings, one can envisage that brain pro-inflammatory cytokines may be considered as a biomarker of AD. This is supported by data in triple-transgenic mice models of AD where targeting the increased circulating levels of proinflammatory cytokine IL-1β with a neutralizing antibody dramatically reduce the activity of several tau kinases and levels of phosphorylated tau (p-tau), and also reduce the load of oligomeric and fibrillar Aβ (fAβ). Thus, it seems that any significant inflammatory response within the brain tissue will be associated with pro-inflammatory cytokines dysfunction, raising the potential use of pro-inflammatory cytokines measure as a surrogate marker for a local inflammatory response in AD.
Dysregulation of pro-inflammatory cytokines in cerebral spinal fluid (CSF)
Altered levels of biomarkers in CSF are supported to be active long before the symptoms appear; moreover the CSF near to the brain parenchyma and the extracellular fluid of the brain. Thus, CSF analysis has been considered an ideal source for viable biomarkers in AD. Currently, the only possible biomarker to aid a diagnosis of AD is the CSF total tau (t-tau) to Aβ42 and phosphorylated tau181 (p-tau181) (53). However, the utility of CSF Aβ and tau as marker may be limited because of its fluctuations over time in CSF or it can be seen in other dementias. Recently, the occurrence of a plaque-dependent inflammation in AD has been extensively documented. There is intense interest in the use of proinflammatory cytokines as biomarkers of AD because they are upregulated during the earliest, oligomeric-induced inflammatory process in AD brains.
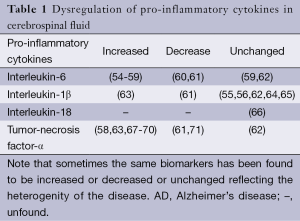
Different cytokines have been measured in CSF such as IL-1α, IL-1β, IL-6, IL-18 and TNF-α, as shown in Table 1, the measurements are very divergent between the different groups. There’s evidence suggesting that IL-1α is as a promising candidate in AD for its important role in disease staging. Pathological altered of IL-1α levels is associated with plaque evolution and the rate of cognitive decline among clinically diagnosed MCI subjects. Advanced studies demonstrated that upregulation of brain levels of IL-1α activity in AD brain is an early event, as IL-1α levels are already significantly increased in MCI CSF, which might represent a novel biomarker of early detection of AD (72,73). Therefore, proinflammatory cytokines levels in CSF are potential biomarkers of disease diagnosis, but remain unrealized.
Full table
Pro-inflammatory cytokine signaling in AD
The deregulation of several pro-inflammatory cytokines has been demonstrated implicated in the pathogenesis of AD. Here we will focus the attention on TNF-α, IL-1, IL-6, IL-12 and IL-18, and speculate their possible roles in AD progression (Table 2).
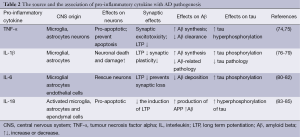
Full table
Tumor necrosis factor alpha (TNF-α)
TNF-α, a pleiotropic pro-inflammatory cytokine, is elevated in both the brains and plasma of AD patients and is proximal to amyloid plaques on autopsy, which appear to be reflective of disease severity and contribute to the inflammatory milieu. As early as 2001, a GWAS found single-nucleotide polymorphisms in TNF-α and/or its receptor is associated with sporadic AD. McAlpine et al. (86) recently reported that when neuron-specific TNF-α is chronically overexpressed in triple-transgenic AD mice (3xTg-AD) using adeno associated virus (AAV) vectors, there is increased intracellular Aβ in the short-term, enhanced inflammation and Tau pathology, and in the long-term that leads to neuronal cell death. The study portends that overexpression TNF-α signaling enhances AD-associated pathology and is detrimental to neuronal viability (87). However, TNF-α is also related to a deleterious role induced by Aβ on promote learning and memory deficits and synaptic memory mechanisms in AD. Inhibition of TNF-α reduced impairment induced by Aβ on recognition memory via long-term potentiation (LTP), electophysiological experiments correlate with learning and memory (88). Meanwhile, the cognitive deficit is reduced by the pharmacological inhibition of TNF-α in mice (89). The results suggest that selectively preventing neuronal TNF-α signaling through targeted blocking of receptor expression, may preserve neurons during the course of AD.
There are two cognate transmembrane receptors for TNF-α, termed TNF receptor 1 (TNFR1) (also known as Tnfrsf1a/p55, CD120a) and TNFR2 (Tnfrsf1b/p75, CD120b). Their biological effects are differentially expressed and regulated. Signaling via the cognate TNF-R receptors elicits distinct cellular responses, including cell proliferation, cell migration, and apoptosis mediated through the activation of several downstream signal transduction cascades involving NF-κB, c-Jun N-terminal kinase (JNK), p38, and ceramide-sphingomyelinase pathways (90). In the context of AD, deletion of the TNFR1 gene in APP transgenic mice reduced plaque deposition and improves the cognitive deficits by down-regulating BACE1 promoter activity. Moreover, McAlpine et al. reported that knock-out TNF-RI in the brains of 3xTg-AD mice can suppress AD-related amyloid pathology. In the same study, short-term inhibiting soluble TNF signaling can prevent AD-associated amyloid pathology using the 3xTg-AD mouse model (86). However, long-term global deleted TNF receptors I and II signaling in the 3xTg mouse model exacerbates hallmark amyloid and neurofibrillary tangle pathology without cell type or stage specificity (91), which suggested that long-term anti-TNF-α should be taken caution. What’s more, the study also suggested that a more selective of TNF signaling and stage of disease should be investigated. Recently, Montgomery et al. found that knockdown of TNF-RII in the long-term in hippocampal neurons via rAAV2 vectore mediated siRNA delivery enhances Aβ plaque deposition and paired helical filament (PHF) formation (90), which have shown TNF-RII may exert protective responses that may be required to counteract TNF-RI driven signal transduction.
However, neuroprotective effects have also been reported for TNF-α. For example, As early as 1995, Barger et al. has found that in the presence of Aβ peptide, dissociated neuronal cultures pretreated with TNF-α were spares cells from Aβ-induced neuronal death by suppressing accumulation of ROS and Ca2+ via NF-κB-dependent signaling (92). Subsequently, TNF-α has also been reported to be trophic to rat hippocampal neurons and to protect against glutamate, free radical, and Aβ toxicity in enriched cultures of primary neurons (93). Moreover, Tarkowski et al. proved intrathecal levels of TNF-α were significantly inversely correlated to the intracerebral apoptosis and neuronal degradation (67). Furthermore, incubation of human neuronal cells with TNF-α led to production of bcl-2, a molecule known to down-regulate neuronal apoptosis. These data indicate the complexity of the TNF signaling pathway, more investigation is deserved to better understand the cell-specific roles of TNF-α in the context of AD.
Interleukin-1β
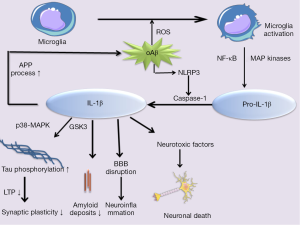
IL-1β, a member of the IL-1 cytokine family, is considered to be a major proinflammatory cytokine in the brain and play a key role in the progress of AD. IL-1β is synthesized and released by both activated microglia and astrocytes in pro-forms, pro-IL-1β, in the cytoplasm in response to variety of stimuli. In order to generate mature and bioactive form, pro-IL-1β must be cleaved by the protease caspase-1, which is activated by cytosolic multiprotein complexes called inflammasomes (94,95). Recently, Parajuli et al. described that soluble oligomeric amyloidβ (oAβ) increased the processing of pro-IL-1β into mature IL-1β in microglia via ROS-dependent activation of NLRP3 inflammation (96).
Previous studies have identified that over-expression of the immune modifying cytokine IL-1β released by microglia and astrocytes surrounding Aβ plaques occur in AD brain and in animal models of AD in relative to age-matched controls (97,98). The production of IL-1β depends on the activation of MAP kinases and NF-κB signaling pathways. Recently, studies clearly demonstrated IL-1β contributing to APP processing in vitro. Subsequently, many researchers confirmed that overexpression of IL-1β exacerbates tau phosphorylation and tangle formation through aberrant activation of p38-MAPK and glycogen synthase kinase 3 (GSK3) (76,99), which affect synaptic plasticity, inhibiting LTP and subsequently learn and memory (100). Blocking or neutralizing IL-1β in an AD mouse model could largely protect from cognitive deficits, decrease tau pathology, synthesis of S100, and fAβ (76). In addition, fAβ has been reported to activate microglia, leading to increased synthesis and release of neurotoxic secretory products, pro-inflammatory cytokines such as IL-1β and ROS (101). Based on these findings, it would be interestingly supposed that Aβ deposits can be both a cause and a consequence of IL-1β expression in AD patients. Some studies have suggested that these two factors participate in a vicious cytokine cycle that once induced and derived AD pathology (Figure 4). In addition, the increased expression of IL-1β, was found to impair microglial Aβ clearance functions (102) and increase BBB permeability, which can promote the accumulation of Aβ in the brain (103). In contrast, evidence points that IL-1β may play a beneficial role in limiting AD pathology. It has been shown that sustained overexpression of IL-1β reduces Aβ-related pathology by modulating microglia-dependent plaque degradation or promoting non-amyloidogenic APP cleavage in a mouse model of AD and in a cell culture model (77,104,105). Thus, IL-1β may play a complex role in AD pathogenesis.
Interleukin-6
IL-6 is a pleiotropic inflammatory cytokine mainly produced by activated microglia, astrocytes in different brain regions. In addition, IL-6 could stimulate microglia and astrocytes to release a cascade of proinflammatory cytokines and acute-phase proteins, such as C-reactive protein (CRP) (106). The levels of IL-6 have been found significantly elevating in the brains, cerebrospinal fluid, and plasma, especially locally around amyloid plaques in AD patients and animal models. Therefore, it has been proposed IL-6 is involved in the etiopathology of AD with acute or chronic inflammatory components.
A number of studies have investigated the molecular mechanism underlying the association of IL-6 with AD including tau and Aβ. Production of IL-6 by human neurons is reportedly stimulated by glycation end product-modified tau and Aβ. Firstly, studies clearly demonstrated the role of IL-6 that contribute to APP processing and production in primary rat cortical neurons (80). In turn, fAβ has been reported can activate microglia, which lead to increase synthesis and release of IL-6 (47). Production of IL-6 depends on the activation of Toll-like receptor 2 (TLR2)-MyD88 signaling pathways in microglia and the JNK-AP1 pathway in human brain endothelial cells (47,107). Secondly, hippocampal cells treated with IL-6 could contribute to NFT formation by inducing Tau phosphorylation through cd k5/p 35 or pathway deregulation. In addition, IL-6 can also activate the JAK/STATs, NMDA receptor and the MAPK-p38 protein kinases, both involved in hyperphosphorylation of tau (80). However, in vivo studies, overexpression of IL-6 in APP transgenic mice and nontransgenic littermates induced by AAV1 leads to marked suppression of Aβ deposition, which does not significantly alter APP levels, APP processing, or steady-state Aβ generation. The date suggest that IL-6-induced neuroinflammation attenuation in Aβ levels instead of exacerbation Aβ plaque pathology, is most likely due to activation of microglia to a predominantly beneficial (M2) phenotype enhanced Aβ phagocytosis (108).
IL-6 in neuroinflammation and neurodegeneration also plays a complex role in regulating cognitive function (109). As early as 2002, Weaver et al. has found that elevated IL-6 correlated with age-related cognitive decline in humans (110). Subsequently, many researchers confirmed that under inflammatory conditions, excessive IL-6 through activation of neuronal NADPH-oxidase induced by aging or inflammation may impair cognitive processes, such as spatial learning and memory (81). By contrast, a recently study reported in cases where inflammation is not prominently elevating IL-6 and/or its downstream JAK/STAT signaling pathway may improve executive function in the mice orbito frontal cortex (111).
Interleukin-18
IL-18 (interferon-γ-inducing factor, IL-1γ), a pro-inflammatory cytokines belonging to the IL-1 cytokine family, is synthesized as an inactive 24-kDa precursor protein (pro-IL-18) and cleaved by the intracellular cystein protease caspase-1 and proteinase-3 to generate a 18 kDa mature and biologically active form. Activated microglia, astrocytes and ependymal cells and neurons in the CNS are all the source of IL-18. Up-regulated IL-18 expression can lead to a harmful vicious cycle of inflammation: IL-18 drives the local production of IL-1β and IFN-γ, the latter cleaves inactive precursor protein of IL-1β and IL-18 to their mature and biologically active form via caspase-1, and it also increases IL-18 gene expression which may play an important role in the pathogenesis of AD (83).
Recently, accumulating date demonstrated that IL-18 may involve in the different aspects of neurodegeneration in AD. Evidences showed that IL-18 possibly has a direct influence on neuronal survival, synaptic plasticity. IL-18 binding to its receptor complex can lead to activation of JNK and MAPK p38, which can activate pro-apoptotic signaling pathways both in intrinsic and extrinsic. The effect seems to be mediated through induction of expression of p53 and Fas ligand (83), which suggests that IL-18 may be one of the apoptosis-inducing factors contributing to AD progression. In addition, studies have shown that IL-18 is cytotoxic to cardiomyocytes, which leads to increased intracellular Ca2+ levels, and calcium dysregulation plays an important role in the pathogenesis of AD (112). More specifically, IL-18 inhibits the induction of LTP in the dentate gyrus, a paradigm for the cellular mechanisms underlying learning and memory (84). However, lower levels of IL-18 related to polymorphisms in the cytokine gene may be associated with improved physical functioning in aged healthy men, which suggested that it may have neuroprotective effects (113). Therefore, a precise involvement of IL-18 in the pathogenesis of AD remains elusive and needs additional investigations.
A number of studies have investigated the molecular mechanism underlying the association of IL-18 with AD pathogenesis, although the exact role of IL-18 in AD still needs to be clarified. In 2012, Sutinen et al. proved IL-18 can increase production of APP and its Thr668 phosphorylation in neuron-like differentiated human SH-SY5Y cells. It can also increase amyloidogenic processing to Aβ by inducing expression of BACE-1 and N-terminal fragment (NTF) of PS-1, part of the functional γ-secretase complex (83), Which suggest heightened or prolonged levels of IL-18 may contribute to the process of AD via increased Aβ. Study conducted by Ojala et al. found that IL-18 may have an impact on the hyperphosphorylation of tau mediated by Cdk5/p35 and GSK-3β kinases (85). Further, increased expression of IL-18 in the brain and peripheral blood associated to cognitive impairment, have been also reported (114). However, a precise involvement of IL-18 in the pathogenesis of AD remains elusive and needs additional investigations.
Pro-inflammatory cytokine modulation as a therapeutic target for AD
Promoted by the substantial science and clinical evidence, pro-inflammatory cytokines is centrally involved in the pathogenesis of AD. Therefore, raises the logical postulation that intervention with drugs targeting modulation microglia proinflammatory cytokine production might be a viable strategy for subverting the disease course. Despite a large body of literature indicating detrimental roles for pro-inflammatory cytokines, neuroprotective effects have also been reported. Thus, strategies to modulate pro-inflammatory cytokines in the disease setting may require selective tuning and specificity to ensure that protective signaling outcomes are not compromised.
Recent studies are focusing on some other pro-inflammatory cytokines modulators such as melatonin, mainly secreted by the pineal gland in mammals, which has been shown to be produced by nonpineal cells and possess anti-inflammatory actions. It reduced the proinflammatory response, decreasing nearly 50% of the Aβ-induced levels of proinflammatory cytokines IL-1β, IL-6, and TNF-α (115). Subsequently experiment reported that melatonin improves the cognitive deficits of rats by inhibiting the local proinflammatory response, mediated by IL-1β induced glial activation in vivo (116). There is also overwhelming studies indicating the potential role of melatonin as an effective adjuvant in AD management (117,118). Unfortunately, a clinical observation indicated essentially negative results after using melatonin in patients with AD (119). Minocycline, a semisynthetic derivative of tetracycline with anti-inflammatory properties, significantly decreased pro-inflammatory cytokines IL-6 and TNF-α released by astrocytes in response to minocycline treatment (120,121). It is previously shown that minocycline treatment reduced the amount of aggregated tau in the cortex of young htau mice and amyloid pathology (122), and improved behavioural symptoms in transgenic mouse models of AD (121,123,124), which may be a potential target to treat AD. In addition, the antirheumatic drug in China xanthoceraside has recently been demonstrated the protective effects on AD pathology by inhibited the Aβ 25-35/IFN-γ–induced pro-inflammatory cytokine NO, IL-1β, and TNF-α in microglia via TLR2/MyD88 pathway to down-regulation of MAPK and NF-kB activities. This study suggesting that xanthoceraside may be a potential therapeutic target for AD treatment (125). More importantly, montelukast a cysteinyl leukotriene receptor 1 (CysLT1R) antagonist, has been used for treatment of inflammatory diseases such as asthma, and currently been proved may ameliorate Aβ-induced memory impairment via inhibiting TNF-α and IL-1β and apoptosis mediated by CysLT1R signaling. Taken together, these results indicated that pro-inflammatory cytokines modulators may be a treatment strategy for AD.
However, some studies are focusing on individual cytokines for therapeutics of AD. Among numerous pro-inflammatory cytokines associated with AD, IL-1β has received particular attention for the role implicated in pathogenic. Blocking IL-1 signaling is able to alter brain inflammatory responses through the reduction of NF-kB activity, markedly reduce tau pathology and partly reduces certain fibrillar and oligomeric forms of amyloid-b in 3xTg-AD mice. Thereby Kitazawa et al. raised the possibility that abrogating IL-1β signaling may offer therapeutic benefit to AD patients (76). In addition, some studies have revealed the relationship between excessive TNF in the brain and the pathological features of AD. By blocking TNF signaling transiently (4 weeks) with soluble TNF-selective dominant negative TNF inhibitor XENP345, amyloid plaques were drastically reduced in the brains of 3xTg-AD mice exposed to chronic systemic inflammation (86). A recent study demonstrated that administration of TNF-α monoclonal antibody (Infliximab) on the AD pathological features in aged APP/PS1 double transgenic mice reduced amyloid plaques and tau phosphorylation (126). Subsequently, many researchers confirmed that administration of TNF-α inhibitor thalidomide in APP23 and 3xTg-AD mice resulted in a significant decrease in the activation of microglia, BACE1 level and activity, Aβ load, plaque formation and tau phosphorylation (127,128). On the contrary, Montgomery et al. reported that long-term knock-down of TNF-RII in hippocampal neurons via rAAV2 vector mediated siRNA delivery enhanced amyloid- and tau-related pathologic features (90). The present work builds on existing data suggested that caution must be taken on selectively modulate TNF signaling in specific cell types and at different stages of disease. Further work is necessary to confirm findings from earlier studies pro-inflammatory cytokine modulation as a therapeutic target for AD.
Conclusions
Increasing evidence has firmly certified that the inflammation induced by Aβ plays a key role in AD pathogenesis. The inflammatory process itself is driven by microglial activation through the induction of pro-inflammatory molecules and related signaling pathways, thus leading to Aβ aggregation, tau formation, synaptic damage, neuronal loss, and the activation of other inflammatory participants. These pro-inflammatory cytokines may have multiple roles in both neurodegeneration and neuroprotection, the use of the beneficial pro-inflammatory cytokine and the control of the detrimental pro-inflammatory cytokine released from microglia depends on our knowledge of their role in AD. However, our knowledge of the intricate role of pro-inflammatory cytokine in AD is far from completion and may lead to variable outcomes. It is critical to get the precise role of pro-inflammatory cytokine in AD, which helps to evaluate the therapeutic value of pro-inflammatory cytokine modulation for AD. Nonetheless, both science and clinical evidences suggest inhibiting pro-inflammatory cytokines therapeutics may be a viable strategy for subverting the disease course. However, strategies to modulate pro-inflammatory cytokines in the disease setting may require selective tuning and specificity to ensure that protective signaling outcomes are not compromised. There are many important questions still need to be answered, such as how different factors and agents modulate pro-inflammatory cytokines signaling in homeostasis and how pro-inflammatory cytokines signaling is intertwined with other innate and adaptive immune pathways. Future studies should focus on in-depth understanding of how they contribute to AD pathology, and it might provide more cues for the development of therapeutic strategies in AD.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China [81471309, 81371406, 81171209], the Natural Science Foundation of Beijing [7152096], the Shandong Provincial Outstanding Medical Academic Professional Program, Shandong Provincial Collaborative Innovation Center for Neurodegenerative Disorders, Qingdao Key Health Discipline Development Fund, and Qingdao Outstanding Health Professional Development Fund.
Disclosure: The authors declare no conflict of interest.
References
- Alzheimer's Association. 2011 Alzheimer's disease facts and figures. Alzheimers Dement 2011;7:208-44. [PubMed]
- Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 2014;88:640-51. [PubMed]
- Glabe CC. Amyloid accumulation and pathogensis of Alzheimer's disease: significance of monomeric, oligomeric and fibrillar Abeta. Subcell Biochem 2005;38:167-77. [PubMed]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002;297:353-6. [PubMed]
- Takahashi RH, Capetillo-Zarate E, Lin MT, et al. Co-occurrence of Alzheimer's disease β-amyloid and pathologies at synapses. Neurobiol Aging 2010;31:1145-52. [PubMed]
- Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des 2010;16:2766-78. [PubMed]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol 2010;6:193-201. [PubMed]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 2004;1:14. [PubMed]
- Jiang T, Yu JT, Tan L. Novel disease-modifying therapies for Alzheimer's disease. J Alzheimers Dis 2012;31:475-92. [PubMed]
- Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer's disease. Neurobiol Dis 2010;37:503-9. [PubMed]
- Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet 2011;43:436-41. [PubMed]
- Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 2011;43:429-35. [PubMed]
- Bertram L, Lange C, Mullin K, et al. Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE. Am J Hum Genet 2008;83:623-32. [PubMed]
- Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 2013;368:107-16. [PubMed]
- Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer's disease. N Engl J Med 2013;368:117-27. [PubMed]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet 2013;45:1452-8. [PubMed]
- Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 2009;64:110-22. [PubMed]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol 2013;39:3-18. [PubMed]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122:787-95. [PubMed]
- Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci 2011;33:199-209. [PubMed]
- Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 2013;493:674-8. [PubMed]
- Zilka N, Kazmerova Z, Jadhav S, et al. Who fans the flames of Alzheimer's disease brains? Misfolded tau on the crossroad of neurodegenerative and inflammatory pathways. J Neuroinflammation 2012;9:47. [PubMed]
- Yang CN, Shiao YJ, Shie FS, et al. Mechanism mediating oligomeric A clearance by naïve primary microglia. Neurobiol Dis 2011;42:221-30. [PubMed]
- Bamberger ME, Harris ME, McDonald DR, et al. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci 2003;23:2665-74. [PubMed]
- Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol 2011;81:825-37. [PubMed]
- Arancio O, Zhang HP, Chen X, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J 2004;23:4096-105. [PubMed]
- Liu YY, Bian JS. Hydrogen sulfide protects amyloid-β induced cell toxicity in microglia. J Alzheimers Dis 2010;22:1189-200. [PubMed]
- Fleisher-Berkovich S, Filipovich-Rimon T, Ben-Shmuel S, et al. Distinct modulation of microglial amyloid phagocytosis and migration by neuropeptides (i). J Neuroinflammation 2010;7:61. [PubMed]
- Ralay Ranaivo H, Craft JM, Hu W, et al. Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci 2006;26:662-70. [PubMed]
- Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflammation 2008;5:25. [PubMed]
- Jaworski T, Lechat B, Demedts D, et al. Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration. Am J Pathol 2011;179:2001-15. [PubMed]
- Solito E, Sastre M. Microglia function in Alzheimer's disease. Front Pharmacol 2012;3:14. [PubMed]
- Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013;155:1596-609. [PubMed]
- Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci 2006;24:167-76. [PubMed]
- Ojala J, Alafuzoff I, Herukka SK, et al. Expression of interleukin-18 is increased in the brains of Alzheimer's disease patients. Neurobiol Aging 2009;30:198-209. [PubMed]
- Abbas N, Bednar I, Mix E, et al. Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J Neuroimmunol 2002;126:50-7. [PubMed]
- Benzing WC, Wujek JR, Ward EK, et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging 1999;20:581-9. [PubMed]
- Prinz M, Priller J, Sisodia SS, et al. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 2011;14:1227-35. [PubMed]
- von Bernhardi R, Tichauer JE, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem 2010;112:1099-114. [PubMed]
- Wang DB, Dayton RD, Zweig RM, et al. Transcriptome analysis of a tau overexpression model in rats implicates an early pro-inflammatory response. Exp Neurol 2010;224:197-206. [PubMed]
- Klein RL, Dayton RD, Diaczynsky CG, et al. Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol Aging 2010;31:2091-102. [PubMed]
- Meraz-Ríos MA, Toral-Rios D, Franco-Bocanegra D, et al. Inflammatory process in Alzheimer's Disease. Front Integr Neurosci 2013;7:59. [PubMed]
- Lee JW, Lee YK, Yuk DY, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation 2008;5:37. [PubMed]
- Sheng JG, Bora SH, Xu G, et al. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol Dis 2003;14:133-45. [PubMed]
- Kitazawa M, Oddo S, Yamasaki TR, et al. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci 2005;25:8843-53. [PubMed]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging 2001;22:837-42. [PubMed]
- Vukic V, Callaghan D, Walker D, et al. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis 2009;34:95-106. [PubMed]
- Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009;73:768-74. [PubMed]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 2007;7:161-7. [PubMed]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 2011;130:226-38. [PubMed]
- Takuma K, Fang F, Zhang W, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A 2009;106:20021-6. [PubMed]
- Hartz AM, Bauer B, Soldner EL, et al. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 2012;43:514-23. [PubMed]
- Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer's disease. Biomark Med 2012;6:455-76. [PubMed]
- Schuitemaker A, Dik MG, Veerhuis R, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging 2009;30:1885-9. [PubMed]
- Gómez-Tortosa E, Gonzalo I, Fanjul S, et al. Cerebrospinal fluid markers in dementia with lewy bodies compared with Alzheimer disease. Arch Neurol 2003;60:1218-22. [PubMed]
- Martínez M, Fernández-Vivancos E, Frank A, et al. Increased cerebrospinal fluid fas (Apo-1) levels in Alzheimer's disease. Relationship with IL-6 concentrations. Brain Res 2000;869:216-9. [PubMed]
- Rösler N, Wichart I, Jellinger KA. Intra vitam lumbar and post mortem ventricular cerebrospinal fluid immunoreactive interleukin-6 in Alzheimer's disease patients. Acta Neurol Scand 2001;103:126-30. [PubMed]
- Jia JP, Meng R, Sun YX, et al. Cerebrospinal fluid tau, Abeta1-42 and inflammatory cytokines in patients with Alzheimer's disease and vascular dementia. Neurosci Lett 2005;383:12-6. [PubMed]
- Galimberti D, Venturelli E, Fenoglio C, et al. Intrathecal levels of IL-6, IL-11 and LIF in Alzheimer's disease and frontotemporal lobar degeneration. J Neurol 2008;255:539-44. [PubMed]
- Yamada K, Kono K, Umegaki H, et al. Decreased interleukin-6 level in the cerebrospinal fluid of patients with Alzheimer-type dementia. Neurosci Lett 1995;186:219-21. [PubMed]
- Hampel H, Teipel SJ, Padberg F, et al. Discriminant power of combined cerebrospinal fluid tau protein and of the soluble interleukin-6 receptor complex in the diagnosis of Alzheimer's disease. Brain Res 1999;823:104-12. [PubMed]
- Lanzrein AS, Johnston CM, Perry VH, et al. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1beta, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-alpha, the soluble tumor necrosis factor receptors I and II, and alpha1-antichymotrypsin. Alzheimer Dis Assoc Disord 1998;12:215-27. [PubMed]
- Llano DA, Li J, Waring JF, et al. Cerebrospinal fluid cytokine dynamics differ between Alzheimer disease patients and elderly controls. Alzheimer Dis Assoc Disord 2012;26:322-8. [PubMed]
- Blum-Degen D, Müller T, Kuhn W, et al. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett 1995;202:17-20. [PubMed]
- Cacabelos R, Barquero M, García P, et al. Cerebrospinal fluid interleukin-1 beta (IL-1 beta) in Alzheimer's disease and neurological disorders. Methods Find Exp Clin Pharmacol 1991;13:455-8. [PubMed]
- Lindberg C, Chromek M, Ahrengart L, et al. Soluble interleukin-1 receptor type II, IL-18 and caspase-1 in mild cognitive impairment and severe Alzheimer's disease. Neurochem Int 2005;46:551-7. [PubMed]
- Tarkowski E, Blennow K, Wallin A, et al. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J Clin Immunol 1999;19:223-30. [PubMed]
- Tarkowski E, Liljeroth AM, Nilsson A, et al. TNF gene polymorphism and its relation to intracerebral production of TNFalpha and TNFbeta in AD. Neurology 2000;54:2077-81. [PubMed]
- Hasegawa Y, Sawada M, Ozaki N, et al. Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology 2000;46:185-8. [PubMed]
- Tarkowski E, Andreasen N, Tarkowski A, et al. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003;74:1200-5. [PubMed]
- Richartz E, Stransky E, Batra A, et al. Decline of immune responsiveness: a pathogenetic factor in Alzheimer's disease? J Psychiatr Res 2005;39:535-43. [PubMed]
- Hu WT, Chen-Plotkin A, Arnold SE, et al. Novel CSF biomarkers for Alzheimer's disease and mild cognitive impairment. Acta Neuropathol 2010;119:669-78. [PubMed]
- Combarros O, Sánchez-Guerra M, Infante J, et al. Gene dose-dependent association of interleukin-1A [-889] allele 2 polymorphism with Alzheimer's disease. J Neurol 2002;249:1242-5. [PubMed]
- Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 2010;22:977-83. [PubMed]
- Zhao J, O'Connor T, Vassar R. The contribution of activated astrocytes to Aβ production: implications for Alzheimer's disease pathogenesis. J Neuroinflammation 2011;8:150. [PubMed]
- Kitazawa M, Cheng D, Tsukamoto MR, et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer's disease model. J Immunol 2011;187:6539-49. [PubMed]
- Tachida Y, Nakagawa K, Saito T, et al. Interleukin-1 beta up-regulates TACE to enhance alpha-cleavage of APP in neurons: resulting decrease in Abeta production. J Neurochem 2008;104:1387-93. [PubMed]
- Griffin WS, Liu L, Li Y, et al. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation 2006;3:5. [PubMed]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci 2008;28:8354-60. [PubMed]
- Spooren A, Kolmus K, Laureys G, et al. Interleukin-6, a mental cytokine. Brain Res Rev 2011;67:157-83. [PubMed]
- Dugan LL, Ali SS, Shekhtman G, et al. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 2009;4:e5518. [PubMed]
- Chakrabarty P, Ceballos-Diaz C, Beccard A, et al. IFN-gamma promotes complement expression and attenuates amyloid plaque deposition in amyloid beta precursor protein transgenic mice. J Immunol 2010;184:5333-43. [PubMed]
- Sutinen EM, Pirttilä T, Anderson G, et al. Pro-inflammatory interleukin-18 increases Alzheimer's disease-associated amyloid-β production in human neuron-like cells. J Neuroinflammation 2012;9:199. [PubMed]
- Curran B, O'Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience 2001;108:83-90. [PubMed]
- Ojala JO, Sutinen EM, Salminen A, et al. Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J Neuroimmunol 2008;205:86-93. [PubMed]
- McAlpine FE, Lee JK, Harms AS, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer's disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis 2009;34:163-77. [PubMed]
- Janelsins MC, Mastrangelo MA, Park KM, et al. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol 2008;173:1768-82. [PubMed]
- Tobinick E. Tumour necrosis factor modulation for treatment of Alzheimer's disease: rationale and current evidence. CNS Drugs 2009;23:713-25. [PubMed]
- Shin JW, Cheong YJ, Koo YM, et al. α-Asarone Ameliorates Memory Deficit in Lipopolysaccharide-Treated Mice via Suppression of Pro-Inflammatory Cytokines and Microglial Activation. Biomol Ther (Seoul) 2014;22:17-26. [PubMed]
- Montgomery SL, Narrow WC, Mastrangelo MA, et al. Chronic neuron- and age-selective down-regulation of TNF receptor expression in triple-transgenic Alzheimer disease mice leads to significant modulation of amyloid- and Tau-related pathologies. Am J Pathol 2013;182:2285-97. [PubMed]
- Montgomery SL, Mastrangelo MA, Habib D, et al. Ablation of TNF-RI/RII expression in Alzheimer's disease mice leads to an unexpected enhancement of pathology: implications for chronic pan-TNF-α suppressive therapeutic strategies in the brain. Am J Pathol 2011;179:2053-70. [PubMed]
- Barger SW, Hörster D, Furukawa K, et al. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci U S A 1995;92:9328-32. [PubMed]
- Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer's disease. Neurobiol Aging 2000;21:383-421. [PubMed]
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 2012;28:137-61. [PubMed]
- Liu L, Martin R, Chan C. Palmitate-activated astrocytes via serine palmitoyltransferase increase BACE1 in primary neurons by sphingomyelinases. Neurobiol Aging 2013;34:540-50. [PubMed]
- Parajuli B, Sonobe Y, Horiuchi H, et al. Oligomeric amyloid β induces IL-1β processing via production of ROS: implication in Alzheimer's disease. Cell Death Dis 2013;4:e975. [PubMed]
- Boutajangout A, Wisniewski T. The innate immune system in Alzheimer's disease. Int J Cell Biol 2013;2013:576383.
- Hunter JM, Kwan J, Malek-Ahmadi M, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer's disease. PLoS One 2012;7:e36893. [PubMed]
- Sheng JG, Zhu SG, Jones RA, et al. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp Neurol 2000;163:388-91. [PubMed]
- Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res 2007;163:339-54. [PubMed]
- Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. ScientificWorldJournal 2012;2012:756357.
- Heneka MT, Nadrigny F, Regen T, et al. Locus ceruleus controls Alzheimer's disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A 2010;107:6058-63. [PubMed]
- Wang Y, Jin S, Sonobe Y, et al. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One 2014;9:e110024. [PubMed]
- Shaftel SS, Kyrkanides S, Olschowka JA, et al. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J Clin Invest 2007;117:1595-604. [PubMed]
- Ghosh S, Wu MD, Shaftel SS, et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J Neurosci 2013;33:5053-64. [PubMed]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329-44. [PubMed]
- Jana M, Palencia CA, Pahan K. Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer's disease. J Immunol 2008;181:7254-62. [PubMed]
- Chakrabarty P, Jansen-West K, Beccard A, et al. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J 2010;24:548-59. [PubMed]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 2011;25:181-213. [PubMed]
- Weaver JD, Huang MH, Albert M, et al. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 2002;59:371-8. [PubMed]
- Donegan JJ, Girotti M, Weinberg MS, et al. A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal cortex. J Neurosci 2014;34:953-62. [PubMed]
- Yu JT, Chang RC, Tan L. Calcium dysregulation in Alzheimer's disease: from mechanisms to therapeutic opportunities. Prog Neurobiol 2009;89:240-55. [PubMed]
- Frayling TM, Rafiq S, Murray A, et al. An interleukin-18 polymorphism is associated with reduced serum concentrations and better physical functioning in older people. J Gerontol A Biol Sci Med Sci 2007;62:73-8. [PubMed]
- Bossù P, Ciaramella A, Salani F, et al. Interleukin-18, from neuroinflammation to Alzheimer's disease. Curr Pharm Des 2010;16:4213-24. [PubMed]
- Rosales-Corral S, Tan DX, Reiter RJ, et al. Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-beta peptide in rat brain: a comparative, in vivo study versus vitamin C and E. J Pineal Res 2003;35:80-4. [PubMed]
- Shen Y, Zhang G, Liu L, et al. Suppressive effects of melatonin on amyloid-beta-induced glial activation in rat hippocampus. Arch Med Res 2007;38:284-90. [PubMed]
- Yang X, Yang Y, Fu Z, et al. Melatonin ameliorates Alzheimer-like pathological changes and spatial memory retention impairment induced by calyculin A. J Psychopharmacol 2011;25:1118-25. [PubMed]
- Spuch C, Antequera D, Isabel Fernandez-Bachiller M, et al. A new tacrine-melatonin hybrid reduces amyloid burden and behavioral deficits in a mouse model of Alzheimer's disease. Neurotox Res 2010;17:421-31. [PubMed]
- Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep 2003;26:893-901. [PubMed]
- Familian A, Eikelenboom P, Veerhuis R. Minocycline does not affect amyloid beta phagocytosis by human microglial cells. Neurosci Lett 2007;416:87-91. [PubMed]
- Garwood CJ, Cooper JD, Hanger DP, et al. Anti-inflammatory impact of minocycline in a mouse model of tauopathy. Front Psychiatry 2010;1:136. [PubMed]
- Corbett A, Williams G, Ballard C. Drug repositioning: an opportunity to develop novel treatments for Alzheimer's disease. Pharmaceuticals (Basel) 2013;6:1304-21. [PubMed]
- Parachikova A, Vasilevko V, Cribbs DH, et al. Reductions in amyloid-beta-derived neuroinflammation, with minocycline, restore cognition but do not significantly affect tau hyperphosphorylation. J Alzheimers Dis 2010;21:527-42. [PubMed]
- Cuello AC, Ferretti MT, Leon WC, et al. Early-stage inflammation and experimental therapy in transgenic models of the Alzheimer-like amyloid pathology. Neurodegener Dis 2010;7:96-8. [PubMed]
- Qi Y, Zou LB, Wang LH, et al. Xanthoceraside inhibits pro-inflammatory cytokine expression in Aβ25-35/IFN-γ-stimulated microglia through the TLR2 receptor, MyD88, nuclear factor-κB, and mitogen-activated protein kinase signaling pathways. J Pharmacol Sci 2013;122:305-17. [PubMed]
- Shi JQ, Shen W, Chen J, et al. Anti-TNF-α reduces amyloid plaques and tau phosphorylation and induces CD11c-positive dendritic-like cell in the APP/PS1 transgenic mouse brains. Brain Res 2011;1368:239-47. [PubMed]
- He P, Cheng X, Staufenbiel M, et al. Long-term treatment of thalidomide ameliorates amyloid-like pathology through inhibition of β-secretase in a mouse model of Alzheimer's disease. PLoS One 2013;8:e55091. [PubMed]
- Tweedie D, Ferguson RA, Fishman K, et al. Tumor necrosis factor-α synthesis inhibitor 3,6'-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. J Neuroinflammation 2012;9:106. [PubMed]


