Intraoperative crisis resource management during a non-intubated video-assisted thoracoscopic surgery
Authors’ introduction:
Figure 1 is part of our team at the University General Hospital of Alicante, and it is composed by thoracic surgeons (Dr. C. Galvez, Dr. S. Bolufer), anesthesiologists (Dr. J. Navarro-Martinez, Dr. MJ. Rivera, Dr. M. Galiana) and the nurses (Mrs. M. Perez, Mrs. E. Ortuño, Mrs. F. Rey). We began on July 2013 with non-intubated uniportal VATS procedures and we are evolving in the anesthetic care, major procedures and fast-track protocols to achieve the least invasive approach for our patients.

Introduction
Introducing a new procedure in a daily routine practice, as is the thoracic surgery, is in many occasions challenging. To be prepared to do it, all the team (surgeons, nurses and anesthesiologists) must have the precise knowledge of what everybody is doing and be aware of the complications that could happen. The use of “awake” anesthesia, or as should be called instead, regional or local anesthesia for thoracic anesthesia, is still a matter of debate on continuous debate (1), despite increasingly encouraging results (2-4). The theoretical benefits are obvious (5); we avoid orotracheal intubation adverse effects of the use a double-lumen tubes, open pneumothorax with a better collapse of the lung and caudal movement of the diaphragm (6). The potential risks include hypoxemia, hypercapnia, cough, severe bleeding and anxiety attacks. All of these complications can evolve into a critical situation where skilled and trained personal is essential. Is it worth the trouble (7)? Or better still can we lower the risk? That’s what we will try to answer.
Critical resource management definition
Managing critical events during a surgery is one of the most challenging and important tasks required of the anesthesiologist and the surgeon. The way to get education and practice in these type of situations is on constant evolution. On the one hand, we have the traditional way of learning based on acquiring knowledge through books and afterwards applying them in the “real world”. On the other hand, we have all the resources available to try to simulate all the critical events before they happen. For that reason, Professor Gaba more than 20 years ago introduced the concept of crisis resource management (CRM) recently reviewed in a new book (8). The CRM refers to the non-technical skills required for effective teamwork in a crisis situation (8), where the patient’s safety during the surgery is the main objective. The concept of CRM was originally developed from the domain of the aviation. Initially it was called “cockpit resource management” and later “crew resource management” due to the importance of simulating all the different scenarios and the group reaction in a crisis.
The CRM is made out from three important words: The first word is “Crisis”, the dictionary define it as the “the turning point of a disease when an important change takes place, indicating either recovery or death.” (9). The second word is “Resource”, “a source of supply, support, or aid, especially one that can be readily drawn upon when needed”. The third word “Management”, “the act or manner of managing, handling, direction or control”. These three words resume very well how our medical knowledge and skills are essential components in the decisions and actions performed during crises, but we have to “see” the entire situation to take control of it. That includes the environment, the equipment and the patient care team. We have to act quickly and safely because the scenario where we work is dynamic and unpredictable. The main objective is the patient’s safety and try to reduce to a minimum the possibility of a human error (10).
Elements of the critical resource management
The key principles of the CRM are:
- Knowing the environment and available resources;
- Anticipate and plan, call for help early;
- Exercise leadership and followership;
- Distribute the workload;
- Mobilize all available resources;
- Communicate effectively;
- Use all the available information;
- Prevent and manage fixation errors;
- Cross (double) check;
- Use cognitive aids;
- Re-evaluate repeatedly;
- Use good teamwork;
- Allocate attention wisely;
- Set priorities dynamically.
The available resources can be categorized as one-self, operating room personal, equipment, cognitive aids, plans, systems, and other external resources. The self-resources are resumed in, our knowledge and our skills. Your performance won’t be constant during the course of the day, will be affected by fatigue, sleep deprivation, emotional disturbance, ill health, inexperience and of course lack of knowledge. The hazardous attitudes and production pressure of the institution must be reduced to a minimum. In order to prevent problems with the equipment we must ensure that this works properly before we start, ensure that critical backup equipment is immediately available (e.g., a self-inflating bag for emergency ventilation), and know how to operate each piece of equipment you use, including knowledge of its operating characteristics in both normal and abnormal circumstances. Included in the resources we have two special important issues: the hazardous attitudes and the production pressure. Examples of hazardous attitudes are: antiauthority “Don’t tell me what to do”, impulsivity “Do something quickly”, invulnerability “It won’t happen to me”, macho “I’ll show you I can do it”, resignation “There’s nothing we can do”. To those attitudes, we have to find antidotes and in some cases, you have to even consult a psychologist. The second is the production pressure, to avoid it you have to have multidisciplinary written consensus guidelines on the preoperative preparation of patients that address the appropriate workup for patients with various medical conditions in different surgical urgency categories. This means, one must adapt the daily surgical program depending on the patients not on the numbers.
The next step is the use of cognitive aids; this type of help is supported by different studies in the literature (11,12). Mnemonic checklists written in all the operation rooms or in the head of every physician are very useful even more during periods of stress. Systems and other external resources include to now “who” we can call for help in case of emergency and “where” the things you need are.
The anticipation and planning of the surgery is essential, even if this is carried out perfectly we will expose the patient to risk. We have to try to expect the unexpected and as the pilots say, “Always fly ahead of your plane”.
The next group of CRM statements can be grouped into “when the crisis happens”. A good teamwork is basic, knowing when to be leader and when to be a follower. The leader has to communicate effectively without raising the voice, state the commands or request as clearly and precisely as possible, avoiding making statements to the air, asking for the confirmation message when you ask for something or someone. The follower must listen what the leader says and do what is needed, but with an open-mind to help and transmit to the leader your concerns.
In an emergency or even a suspected emergency calling for help early is mandatory. When you are a junior physician, you will do it many times. It’s your duty to do it and it’s not a signal of low self-confidence. You must know what type of help you need: muscle, transport, general technical skill, thinking or just someone that you feel confidence.
A specific type of error in these situations is the fixation errors. This type of error is very common in dynamic settings and what creates it is the persistent failure to revise a diagnosis or plan in the face of readily available evidence that suggests a revision is necessary. The three most common are the following situations: “This and only this”, not taking into account an alternative diagnosis, “Everything but this”, neglecting one diagnosis, “Everything is ok”, do not recognize the need to act in emergency mode.
Try to prevent these errors, cross (double) checking all the actions you perform and re-evaluation from the beginning. Even if you think you’ve done it properly. Acute medicine is dynamic. What is correct now may be wrong the next minute.
Use good teamwork, for that reason you have to build it before the situation begins. Every member should be prepared to know what the other team member is going to do. Allocate attention wisely to do it properly you may use two types of actions: (I) try to develop a sequence of actions, as the one used during a CPR; (II) try to alternate between focusing on details and focusing on the big picture.
Set priorities dynamically, as we said before, acute medicine is dynamic. The decision you make, it’s your decision and it could not be the best decision. However, your main priority is ensuring stable signs at all the time. Let all the team to know them also.
Application of the critical resource management in NI-VATS
Knowing the environment and available resources
Self-resources
In the first place, always-experienced team performs the NI-VATS. To define experienced team we use the following criteria, the surgeons, anesthesiologists and OR nurses must have done more than 50 conventional VATS, overcome the learning curve in conventional multiportal VATS and uniportal VATS (more than 50 major procedures through each approach) and have experience with difficult cases of major lung resections [big and central tumors, broncho-angioplasties, tumors of the apex, tumors with invasion of surrounding structures (diaphragm, chest wall, pericardium)], and management of complications such as moderate to severe bleeding through the VATS or single-port approach. In addition, the anesthesiologist must be trained in the lateral intubation at least in 20 cases (left and right). This is very important; in case of a crisis, one must know how to intubate in the lateral position. During the regular training this type of technique is not done, for that reason before we started doing NI-VATS we trained ourselves in scheduled patients.
Lateral intubation special issues
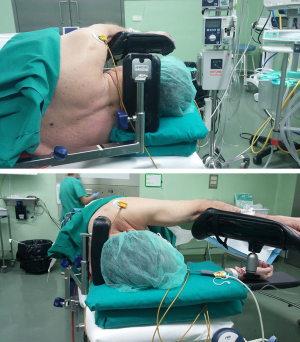
The first thing to know is that this is not more difficult than do it in the supine position. Second, the lateral position of the patient is very useful for the entire team. The patient can put himself in the most comfortable position (preventing iatrogenic stretching injuries of the brachial plexus). The surgeon can obtain the best surgical approach without harming. The anesthesiologist can intubate without increasing the risk. The nurse doesn’t have to move the entire heavy weighted patient once he is asleep. Theoretically, everybody is happy. How is the correct way to do it? As we can see in Figure 2, the head must be in a neutral position, using a couple of surgical pillows and an occipital support to prevent that the head goes backwards during the laryngoscopy. Ventilation is more easily performed than in supine position, actually this position is the safe position to prevent lung aspiration and the hypopharyngeal structures won’t easily cause an airway obstruction. Correct manual ventilation is normally achieved without the need of Guedel airway, regardless of the side to intubate. Laryngoscopy and intubation in right sided position are usually more hazardous compared to the left sided. This is because it is more difficult to direct the double lumen tube properly, even if normally we can do it without help of intubating introducers or similar. Once the double lumen tube is inside the trachea, it is generally well positioned the first time. We have to pay particular attention to patients with predictive criteria of difficult airway; those patients are not excluded from the NI-VATS (13), in which we directly use the Airtraq® videolaringoscope and the bronchofiberscope.
The hazardous attitudes and the production pressure
To solve this problem not all the surgeons or anesthesiologists can perform a NI-VATS, only the ones that knows their own limitations and try to improve them. The NI-VATS is scheduled the first case of the day.
The equipment
The surgical equipment we use is the standard VATS. We prepare a special equipment in case we have to convert into an emergency thoracotomy or general anesthesia, it’s called “emergency table” and is composed by following items (Table 1): thoracic drainage (24 Fr), two sizes of double-lumen tubes [35-37], two sizes of single lumen orotracheal tubes (7 and 7.5 internal diameter), Guedel cannula, medication to induce a general anesthesia (200 mg of propofol, 300 mg of fentanyl and 100 mg of rocuronium), macintosh laryngoscope with two blade sizes and the Airtraq® videolaringoscope. We also have prepared and ready to use the bronchofibroscope.

Full table
Cognitive aids
We use a checklist described in Table 1, all items must be fulfilled for a NI-VATS. We are currently in the process of developing the entire different intraoperative crisis checklist to have them in the operation room.
Systems and other external resources
In our hospital at the beginning of the case, we tried to be two anesthesiologists and two surgeons (as usual). In addition, we have a colleague to call in case of an emergency.
Anticipation and plans
In order to try to prevent patient related problems we have a strict selection protocol. The surgeon and the anesthesiologist explain the advantages and disadvantages of the NI-VATS to the patient. In case of doubt, we exclude him from the surgery and a conventional surgery is undertaken. We follow our checklist.
Exercise leadership and followership
If a surgical emergency happens (severe bleeding) and the patient is stable the leader is the surgeon. If a medical emergency happens (oxygen desaturation), the anesthesiologist takes control of the situation and can make the surgery stop. This is important to allocate the other human resources (nurses). When a NI-VATS is undertaken we have three nurses in the operation room. If an emergency happens, one nurse works with the surgeon and the other works with the anesthesiologist. In our hospital we don’t have anesthesia specific nurses so the surgical nurse helps.
Call for help — prevent and manage fixation errors
When we started to do the NI-VATS, we were two anesthesiologists in the operation room. Nowadays there are always two at the beginning and one of our colleagues in the surgical area in case of an emergency. To prevent fixation errors, in case of a crisis, we always call an expert surgeon or anesthesiologist.
Use good teamwork
All the members of the team know what to do in every moment. We have had several briefing meetings before we started to do it.
Allocate attention wisely—set priorities dynamically
To train these skills we have a manikin (SimMan® Laerdal) where to simulate different crisis situations. The simulation is one of the bases of CRM.
Clinical and critical situations
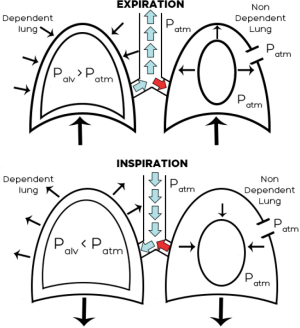
To understand what situations we can find ourselves first we have to know the pathophysiology of the surgical pneumothorax in the NI-VATS (6). The collapse of the non-dependent lung starts once the atmospheric air enters the pleural cavity. Depending on the size of the chest opening, the degree of the collapse can be bigger o smaller. If the patient is in the lateral position, breathing spontaneously and the pneumothorax induced two circumstances, we have to bear in mind two things (Figure 3). First, mediastinal shift towards the dependent lung, due to the loss of negative pressure of the non-dependent lung. Second, paradoxical respiration, during inspiration the non-dependent lung collapses and expands during expiration. Both changes decrease the efficiency of the spontaneous ventilation with re-breathing of exhaled gases. This impaired respiratory function can be aggravated due to the reduced activity of the intercostal muscle function following the epidural anesthesia and the excess of sedation needed for the patient.
Respiratory acidosis
The permissive hypercapnia is very common in the NI-VATS, typically is well tolerated. The reason of this is not fully understood, it could be explained by hypoventilation produced with the changes before explained. When we first started, we used to use the nasal cannula to monitor CO2 but we found an extremely big difference between the exhaled and arterial CO2. Even in the same patient and depending on the degree of the collapse, the variation wasn’t lineal. For that reason, we decided to take them out of the protocol. Nowadays we monitor directly pH, PCO2 and PO2 through the invasive motorization of blood pressure using the radial artery. In our experience, we have never had to treat a severe hypercapnia. In case we would need it, we always have a non-invasive ventilator prepared.
Hypoxemia
The degree of hypoxemia varies a lot depending of the type of patient. As we all see in our thoracic patients the PaO2 arterial drop is much severe in “healthy patients” (14), those without preexisting lung problems related to ventilation and perfusion mismatching (15,16). During the surgery, our objective is to maintain oxygen saturation over 95%. This is normally reached with the administration of oxygen through a Venturi mask. We even had patients without the need of supplemental oxygen during the procedure. In case of a severe hypoxemia we first use a high flow oxygen ventilation (Optiflow®) with the lower flow that increases the volume of the non-dependent lung, usually 20-30 L/min and 100% oxygen fraction. If the patient is still hypoxemic, we can use the non-invasive ventilation. In our experience, if the patient does not tolerate the high flow ventilation, then the need to intubate in the side position is the only solution.
Dyspnea
At the beginning of the procedure, when the pneumothorax is done, they can feel dyspnea, even without a drop of oxygen saturation. We explain it to the patient in the preoperative evaluation and we repeat it before the pneumothorax is undertaken. If the patient knows this situation can occur it is far better tolerated. If the dyspnea continues, we then sedate the patient.
Cough
One of the very usual problems during the NI-VATS is the cough of the patient. Cough receptors are located mainly on the posterior wall of trachea, pharynx, and mucosa of bronchus. Impulses caused by stimuli travel via the vagus nerve to the medulla of the brain, and trigger a cough. The predominance of vagal tone after sympathetic block by TEA might potentially increase bronchial tone and reactivity.
There are several ways to block this coughing reflex, but none of them really suppress it 100% without risks: The first option is to administrate 1-2 mg/Kg of lidocaine and then a continuous perfusion, but the risk of reaching the local anesthetic toxicity is very near. The second way is to use inhalation of aerosolized 2-4% lidocaine in a high oxygen flow for about 30 min before the surgery. The third option is to do an intrathoracic vagal block with 2 mL of 0.25% bupivacaine adjacent to the vagus nerve (17) under direct thoracoscopic vision at the level of the azygous vein in the right side, and just below the aortopulmonary window in the left side, in order to avoid laryngeal recurrent nerve palsy. It has the risk of affecting heart rate, breathing rate, and blood pressure. This option has one variant, which consists on aerosolizing Bupivacaine 0.25% over the visceral pleura and the posterior mediastinal pleural through the vagus nerve course. The last one is to block the ipsilateral stellate ganglion with 10 mL of 0.25% bupivacaine. This aspect is on continuous evolution, what we usually do is a mixture of all the options. We start aerosolizing lidocaine 30 min before the surgery to anesthetize the tracheobronchial tree, and then we instill directly the intrathoracic vagus nerve or aerosolize the visceral and posterior mediastinal pleura with Bupivacaine. If with those maneuvers we do not obtain good results, we then add intravenous lidocaine to upgrade the effect.
Anxiety attack
To prevent it we evaluate the patients carefully. In case of doubt, we exclude it from the program. If during the surgery the patient starts with anxiety symptoms, we use midazolam.
Massive bleeding
In our protocol, the patient has two peripheral venous catheters in the contralateral side of the surgery. This is because in case of an emergency we would use one of the catheters to introduce a rapid infuse catheter set (RIC®Teleflex®) in case we need to infuse fluids rapidly. On a regular basis we don’t cannulate central venous catheters, only after the patient is intubated and with the RIC inserted.
Criteria for a conversion to VATS multiportal, thoracotomy and/or general anesthesia
We use the criteria listed in Table 2. It is important to distinguish between a surgical or a medical crisis. In the medical crisis (usually cough, anxiety or in some cases hypoxemia) we induce general anesthesia, intubate the patient and introduce thoracic drainage through the incision. Once done, we connect it to water seal system and close the wound with a sterile transparent dressing around the drainage, to let the lung reexpand again and improve oxygen saturation or dyspnea. The main causes are:

Full table
- Respiratory acidosis with pH <7.1, with taquipnea (higher than 30 rpm);
- Hypoxemia (PO2 <60 mmHg) with no improvement despite high flow oxygenation nor non-invasive ventilation;
- Continuous cough with no improvement despite aerosolized lidocaine nor vagal blockade;
- Anxiety attack with no improvement with sedation;
- Patient voluntary desire of conversion.
If the crisis is surgical (bleeding), we have less time to react and the anesthesiologist tries not to ventilate the patient until the double lumen tube is not correctly positioned (checked using the bronchofibroscope). We do it this way otherwise the ventilation of the non-dependent lung in the uniportal NI-VATS would make the correction of the emergency very difficult.
We usually perform awake non-intubated procedures through a uniportal approach with a single incision between 4-5 cm length. The main reason for using this approach is that it’s the most used for our pulmonary major and minor resections because it reduces the pain to just a single intercostal nerve and allows a more anatomical view of the hilar structures and the pleural cavity. However, it also proves to be useful because its bigger size facilitates surgical pneumothorax compared to a thoracoscopic port, allowing easier manipulation of the lung in a patient with diaphragmatic motion and mediastinal displacement preserved.
Should a complication happen during the procedure, we choose if we need conversion to multiportal VATS or open thoracotomy.
Reasons for conversion to multiportal VATS
Usually these situations may challenge the surgeon’s skill to solve it through a single incision but do not need emergent maneuvers:
- Extense adhesions of the lung to the chest wall in more than 50% of its surface in a spontaneously breathing patient;
- Big tumors, particularly in the most central and anterior lung that make difficult to manipulate the hilar structures through the single incision;
- Non-adequate lung collapse which difficult lung mobilization;
- Bronchoplasties (wedge or sleeve techniques);
- Mild bleeding (small pulmonary artery and vein branches, bronchial arteries).
With thoracoscopical view through the incision, one or two more thoracoscopic ports are designed. In our VATS multiportal approach we set one of the ports in the same anterior axillar line but several intercostal spaces below, and the third one more posterior in the line of the tip of the scapulae (2 or 3 spaces above the diaphragm), but at the same horizontal line with the previous secondary port, creating a triangle shape. We use a thoracoscopic port if we are introducing the thoracoscope through it, but if we only need endograspers to handle the lung we just perform the incision and introduce them without port, in order to avoid intercostals nerve traumatism.
Reasons for conversion to an open thoracotomy
Reasons for conversion to an open thoracotomy are more severe and challenging, and need that the nurse team, anesthesiologist and the surgeons work coordinately, efficiently and fast, without space for a mistake. The non-intubated patient is, at least, partially aware of what the team says and what is happening, and also has no muscular relaxation and this can aggravate the situation, especially if anxiety develops. Situations that require open thoracotomy usually require also general anesthesia conversion, but not all of them:
- Moderate to major bleeding, uncontrollable with the single incision/awake procedure, which requires more important maneauvers (pulmonary artery clamp, primary suture, reconstruction);
- Impossibility of nodule palpation through the single-incision in the awake patient (small nodules, central location) in the case we do not have a percutaneously set hook for guidance.
If the complication is life threatening and we cannot assure conversion to general anesthesia delaying the surgical response in order to preserve a uni/multiportal VATS approach, then we need to temporarily control it while conversion by the anesthesiologist is initiated, and meanwhile opening the single incision anterior and posteriorly as quick as possible to put a Finochietto retractor. The main cause for this emergent global conversion is major bleeding, which needs compression through a sponge-stick through the incision while converting. After the thoracotomy is opened, we can remove the compressing sponge-stick and control the bleeding by means of harmonic or bipolar cautery, primary suture, pulmonary artery clamping or stapling the vessel.
On the other hand, we have situations where we need to enlarge the utility incision scarcely but conversion to general anesthesia is not mandatory, such as small-undiagnosed nodules or central lesions sometimes cannot be palpated through a small incision if a hook has not been foreseen preoperatively. We then need to enlarge barely the utility incision in order to introduce our fingers.
Many surgeons are afraid of the potential complications of non-intubated procedures. In our experience, after developing the skill in many minor and major uniportal VATS surgeries, the main issue for initiating a non-intubated program requires strict and adequate selection of the patients depending on their pathologies, but also their anatomical and psychological features. Developing an Emergency Protocol as we did is the second step to systematize the action should a problem occurs. The third step, lies on the confidence between the surgical, anesthetic and nursing teams.
Conclusions
The surgical and medical emergencies during a non-intubated video-assisted thoracoscopic surgery must follow the CRM principles. Knowing the environment and available resources, anticipating, planning and using cognitive aids are the basis to minimize the risk of complications. Even if we do it perfectly, crisis occurs, for that reason it is mandatory calling for help early, exercise leadership and followership, distribute the workload, mobilize all available resources, communicate effectively, prevent and manage fixation errors, cross (double) check, use cognitive aids, re-evaluate repeatedly, use good teamwork, allocate attention wisely, set priorities dynamically. The only way to maintain or increase our patient’s care is to practice and train them, with all the team involved in the care.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pompeo E, Mineo TC. Awake thoracic surgery: a historical perspective. In: Pompeo E. eds. Awake thoracic surgery.Sharja: U.A.E. Bentham Sci Pub, 2012:3-8.
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Kiss G, Claret A, Desbordes J, et al. Thoracic epidural anaesthesia for awake thoracic surgery in severely dyspnoeic patients excluded from general anaesthesia. Interact Cardiovasc Thorac Surg 2014;19:816-23. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Dauri, M, Celidonio, L, Nahmias, S, et al. Adverse effects of general anesthesia in thoracic surgery. In: Pompeo E. eds. Awake thoracic surgery, London: Bentham Sci Pub, 2012:34-42.
- Pompeo E. Pathophysiology of Surgical Pneumothorax in the Awake Patient. in: Pompeo E. eds. Awake Thoracic Surgery (Ebook). Sharja: U.A.E. Bentham Sci Pub, 2012:9-18.
- Pompeo E. Awake thoracic surgery--is it worth the trouble? Semin Thorac Cardiovasc Surg 2012;24:106-14. [PubMed]
- Gaba DM, Fish KJ, Howard SK, et al. eds. Crisis management in anesthesiology. 2nd edition. Philadelphia, PA: Elsevier/Saunders, 2015.
- Crisis: definition of crisis in Oxford dictionary (British & World English) [Internet]. Accessed on Jan 22th, 2015. Available online: http://www.oxforddictionaries.com/definition/english/crisis
- Reason J. Human error: models and management. BMJ 2000;320:768-70. [PubMed]
- Arriaga AF, Bader AM, Wong JM, et al. Simulation-based trial of surgical-crisis checklists. N Engl J Med 2013;368:246-53. [PubMed]
- Marshall S. The use of cognitive aids during emergencies in anesthesia: a review of the literature. Anesth Analg 2013;117:1162-71. [PubMed]
- Galvez C, Bolufer S, Navarro-Martinez J, et al. Awake uniportal video-assisted thoracoscopic metastasectomy after a nasopharyngeal carcinoma. J Thorac Cardiovasc Surg 2014;147:e24-6. [PubMed]
- Lohser J. Managing hypoxemia during minimally invasive thoracic surgery. Anesthesiol Clin 2012;30:683-97. [PubMed]
- Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology 2015;122:932-46. [PubMed]
- Ishikawa S, Lohser J. One-lung ventilation and arterial oxygenation. Curr Opin Anaesthesiol 2011;24:24-31. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]


