The complex care of severe emphysema: role of awake lung volume reduction surgery
Authors’ introduction:
Figure 1 is a picture including the authors of the article with other representatives of the ATSRG, a multidisciplinary team aimed at accomplishment and promotion of pioneering and comprehensive clinical investigations focused on awake and nonintubated thoracic surgery.

Introduction
Emphysema, one of the chronic obstructive pulmonary disease (COPD) phenotypes, is an irreversible, progressively debilitating condition, which is estimated to account for about one third of all diagnoses of COPD (1). Due to continuing use of tobacco and biomass fuels as well as to aging populations, emphysema is associated with a global social and economic burden and it is projected to become within 2030 the third leading cause of death (2-4).
In advanced stages, emphysema reduces both lung elastic recoil and mechanical support of peripheral bronchioles eventually leading to early expiratory airway collapse, severe gas trapping and lung hyperinflation.
Standard management of emphysema entails smoking cessation, pharmacological treatment with bronchodilators and anti-inflammatory drugs, supplemental oxygen and rehabilitation.
However, in patients with severe emphysema, medical therapy is poorly effective and interventional treatment including lung volume reduction surgery (LVRS) can be considered.
The standard LVRS procedure, which entails nonanatomic resection of destroyed lung regions through general anesthesia with single-lung ventilation (resectional LVRS) (5), has shown to offer significant and long-lasting improvements in respiratory function, exercise capacity, quality of life and survival, particularly in patients with upper-lobe predominant emphysema and low exercise capacity (6). Unfortunately, several complications can occur with resectional LVRS leading to mortality and morbidity rates as high as 5% and 59%, respectively (7).
These figures, have led to a reportedly underuse of resectional LVRS and have stimulated investigation towards less invasive surgical and bronchoscopic (8-10) nonresectional methods that could assure equivalent clinical results with fewer morbidity.
In order to overcome the main drawbacks of resectional LVRS, we have developed an original nonresectional LVRS method, which can be performed in fully awake patients (11). Clinical results of this ultra-minimally invasive procedure have been highly encouraging (12) and in a uni-center randomized study, intermediate-term outcomes paralleled those of resectional LVRS with shorter hospital stay and fewer side-effects (13).
In this article we analyze indications, technical details and results of awake LVRS taking into consideration the available data from the literature.
Background
Resectional LVRS
Resectional LVRS was initially proposed by Brantigan and Mueller in 1957 (14). The procedure entailed non-anatomic resection of emphysematous lung tissue performed through staged thoracotomy and was aimed at reducing the overall lung volume to re-configurate the chest wall and diaphragm, and restore radial traction on the bronchi, thereby relieving expiratory flow obstruction. At that time, despite significant subjective benefit occurred in most of patients, the lack of objective benefit documentation and a high mortality rate of 18% (15), led to rapid abandonment of LVRS. Following a dormant phase of 4 decades, LVRS was eventually re-vitalized by Cooper and coworkers (5) who proposed technical refinements including the use of median sternotomy for simultaneous bilateral, staple resection of the lungs. In a first series this method resulted in no mortality and significant improvements in subjective dyspnea, pulmonary function, exercise tolerance and quality of life measures. Thereafter, similar satisfactory results have been reproduced by means of video-assisted thoracic surgery (VATS) LVRS performed either unilaterally (16-18) or bilaterally (19-21).
The large National Emphysema Treatment Trial (NETT) (6) confirmed greater and long-lasting benefit as well as a survival advantage of resectional LVRS when compared to maximized medical therapy, particularly in patients with upper-lobe predominant emphysema and low exercise capacity. However, in the NETT operative mortality was 5% and overall morbidity was 59% (7). Time spent for postoperative recovery was often prolonged with about 30% of patients being still hospitalized or in rehabilitation facilities 1 month after surgery. As a result, the cost-effectiveness of LVRS has been questioned (22) progressively leading to a generalized underuse of this procedure in recent years (23).
Nonresectional LVRS by awake anesthesia (awake LVRS)
Historical background of nonresectional LVRS with lung plication can be dated back to the Brantigan’s work in which both resection and plication of emphysematous lung tissue was already described to achieve an adequate reduction in the lung volume (24).
In 1992, Crosa-Dorado et al. (25) proposed multiple fold plications of emphysematous bullae carried out by thoracotomy with the aid of a custom-made folding forceps. In 1998, Swanson and co-workers (26) slightly modified the method proposed by Crosa-Dorado to make it suitable for VATS application. A further original fold plication method has been proposed in 1999 by Iwasaki et al. (27).
All the previously mentioned nonresectional LVRS procedures entailed use of general anesthesia with single-lung ventilation.
In 2006, we (11) reported feasibility and early results of an original nonresectional LVRS technique entailing introflexive plication of the most emphysematous lung regions, which was developed by one of the authors (EP) to be ideally performed in spontaneously ventilating awake patients through thoracic epidural anesthesia (TEA). This method respected the basic concepts of resectional LVRS including a reduction of about 30% of the lung volume, suturing performed along a single ideal line and use of stapling devices. Yet, it added potential advantages including avoidance of any loss in lung tissue, peripheral, interrupted suturing which was hypothesized to be more flexible, avoidance of any pleural discontinuation and creation of inlay buttress by the plicated bullous tissue. These technical refinements were aimed at facilitating immediate postoperative re-expansion of the lung and at reducing risks of prolonged air leaks, which accounts as the most frequent side-effect of resectional LVRS.
Selection criteria
Inclusion and exclusion criteria for awake LVRS do not differ significantly from those of resectional LVRS (28) (Table 1).

Full table
Patients complaining of disabling dyspnea with moderate-to-severe obstructive defect and limited exercise capacity that are not reversed by maximized medical therapy, with radiologic evidence of lung hyperinflation hyperinflation and flat diaphragms on chest X-ray, are potential candidates and must undergo high-resolution computed tomography (HRCT), assessment of static lung volumes by body plethysmography, and of diffusing capacity for carbon monoxide (DLCO).
Optimal candidates for awake LVRS disclose increased residual volume (RV) on body plethysmography and heterogeneous, severe emphysema on HRCT. In particular, we have found that as already shown with non-awake LVRS, patients with upper lobe-predominant emphysema are ideal candidates for awake LVRS and those who can achieve the greater magnitude of improvements (Figure 2). However, patients with lower-lobe predominant heterogeneous disease also can meaningfully benefit from the awake procedure (Figure 3).
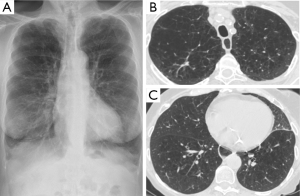
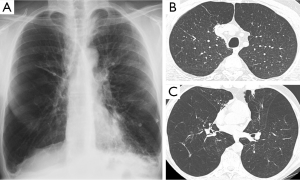
On the other hand, the finding that forced expiratory volume in one second (FEV1) ≤20% predicted and either homogeneous emphysema or DLCO ≤20% predicted, resulted in a mortality rate of 16% following resectional LVRS, has led to consider patients with these characteristics as non-eligible for the operation (29) even though results achieved by the awake LVRS in this patients’ subgroup has not yet been reported.
Moderate hypoxemia is commonly found in candidates for awake LVRS and does not represent an exclusion criterion.
Stable abstinence from cigarette smoking is mandatory to minimize operative risks and can help confirm the patient’s motivation to undergo the operation.
Specific contraindications for awake LVRS include morbid obesity, unwillingness to undergo an awake surgical procedure, excessive anxious symptoms or HRCT findings showing signs of an obliterated pleural cavity on side chosen for LVRS.
Anesthesia
The main differences between awake and nonawake anesthesia are summarized in Table 2.
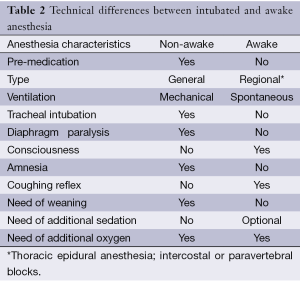
Full table
Our preferred type of anesthesia for awake LVRS is TEA carried out in fully awake, spontaneously ventilating patients. The objective of TEA is to achieve somatosensory and motor block between the T1-T8 level while preserving diaphragmatic motion.
The epidural catheter is inserted at T4-T5 level. During the procedure, the anesthetic regimen entails continuous infusion of ropivacaine 0.5% and sufentanil 1.66 µg/mL into the epidural space whereas supplemental oxygen is delivered through a Venturi mask to maintain oxygen saturation above 90%.
At end-procedure, the anesthetic regimen is changed to ropivacaine 0.16% plus sufentanil 1 µg/mL at 2-5 mL/h and the epidural catheter is removed on the second postoperative day.
In patients with spinal deformity or coagulation disorders contraindicating the use of TEA, awake LVRS is accomplished through paravertebral (30) or intercostal blocks.
Conversion to general anesthesia is considered in patients showing poor tolerability of an awake procedure or whenever unexpected operative findings or technical difficulty are deemed better manageable by general anesthesia. This is induced by intravenous propofol (1.5-2 mg/kg), fentanyl (0.1 mg) and vecuronium (0.1 mg/kg) and is maintained by fentanyl and vecuronium with a continuous infusion of propofol. A left-sided double-lumen tube is used for single-lung ventilation. Intraoperative conversion from awake to general anesthesia is routinely carried out without changing the patient position and with the aid of a videolaryngoscope and a fiberoptic bronchoscope to facilitate tracheal intubation and obtain a correct position of the double lumen tube, respectively.
After surgery, patients undergoing awake LVRS stay in the recovery room for about 30 min and are then directly transferred to the ward where they can immediately start drinking, eating and walking under physiotherapist assistance.
Surgical technique
The patient position is lateral decubitus as for thoracotomy. The operating table is usually not flexed below the chest to facilitate ventilation of the dependent lung. The video monitor is placed at the head of the table. Surgical access entails placement of four flexible trocars. The camera port is placed in the sixth intercostal space along the midaxilllary line while operating ports for instrumentation are placed in the third and/or fifth intercostal space along the anterior axillary line, and in the fourth intercostal space along the posterior axillary line. A 30°, 10 mm camera is used to optimize vision during spontaneous ventilation. Pleural adhesions, if present, are divided by sharp and blunt dissection.
The goal of nonresectional LVRS is to plicate as much emphysematous tissue as possible. The most destroyed lung regions targeted for plication on the basis of the HRCT imaging are recognized intraoperatively with the aid of instrumental palpation of the lung.
Whenever, the hyperinflation of the lung counteracts the lung collapse induced by creation of the surgical pneumothorax, we employ an endopaddle to push down the lung and improve exposure and surgical manoeuvring.
Subsequently, the apical side of the emphysematous target area is grasped by two ring forceps while pushing downward the tissue in between with a cotton swab. The next step entails simultaneous grasping of both redundant lung edges and peripheral suturing of the plicated area by a 45 mm, non-cutting endoscopic stapler. In a similar manner, two other cartridges are fired in the ventral and dorsal side of the targeted area to perform a linear, interrupted suture line. As a result, the upper lobe volume is reduced by about 50% without any loss in lung tissue and the lung is remodelled to achieve a trapezoidal shape. In patients with lower lobe predominant emphysema, multiple smaller plications are carried out to reduce in a uniform manner the overall lung volume (Figure 4).
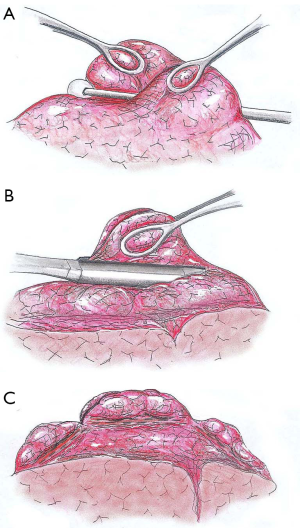
The main differences between resectional and nonresectional LVRS are detailed in Table 3.
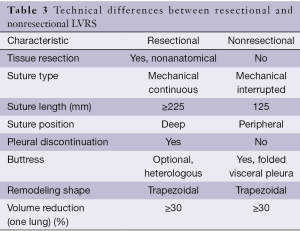
Full table
Surgical strategy
Simultaneous bilateral LVRS has shown to produce superior benefit than unilateral treatment and is the preferred strategy of treatment in several Institutions (31,32). However, we have reported that in patients with asymmetric emphysema undergoingt unilateral LVRS on the most emphysematous lung, the improvement in FEV1 compared that achieved by one-stage bilateral treatment (33,34). This is possibly due to mobility of the mediastinum, which can induce interdependence effects leading a single procedure to improve ventilation in both lungs.
Moreover, it has been shown that yearly deterioration in FEV1 was greater following bilateral than after unilateral LVRS (35). These features summed up to the easier patients’ tolerability of unilateral rather than bilateral simultaneous treatment, have led us to prefer a staged bilateral strategy of treatment. In fact, although there are no prospective studies comparing staged vs. 1-stage bilateral LVRS, in a previous retrospective analysis by our group, staged bilateral LVRS resulted in more stable improvements in FEV1, forced vital capacity (FVC), 6-minute walking test (6MWT) and RV than 1-stage bilateral LVRS (36).
As a result, our current strategy of treatment entails unilateral awake LVRS performed initially on the most severely emphysematous lung and postponement of the contralateral procedure until when the benefits achieved by the first operation are lost.
In our hands, more than 95% of awake LVRS procedures are performed by VATS whereas we deserve thoracotomy to patients with history of previous major thoracic surgery on side chosen for LVRS, in those with radiologic signs of diffuse, fibrous adhesions as well as whenever unexpected intraoperative findings or complications lead us to consider convertion to thoracotomy the safest choice.
Results
As far as perioperative outcome of awake LVRS is concerned, in a comparative analysis, 66 patients undergoing awake nonresectional LVRS were compared with 66 patients undergoing non-awake resectional LVRS. Prolonged air-leak (>7 days) occurred in 18% of the patients in the awake group vs. 40% in the control group (P=0.007) with an overall duration of 5.2 days in the awake group and of 7.9 days in the control group (P<0.0002). As a consequence hospital stay was 6.3 vs. 9.2 days, respectively (P<0.0001) (37).
Clinical benefits of awake LVRS are expected to be equivalent to those achievable by resectional LVRS and include improvements in respiratory function, exercise capacity, subjective dyspnea, quality of life measures and survival (38). Other less frequently reported benefits include improvements in oxygenation (39), body weight and nutritional status (40), cardiac function (41,42), cognitive function (43), alveolar ventilation (44), and breathing pattern (45).
So far there exist only a limited number of publications reporting on the intermediate-term results of awake LVRS.
In a 42 patients retrospective series, we reported no 90-day mortality with significant 2-year improvements in 6MWT, FEV1, FVC, RV as well as in the multidimensional body mass index, airflow obstruction, dyspnea, and exercise capacity index (BODE), which has shown to represent a useful predictor of survival in COPD patients (12).
In a more recent study, 63 patients were randomized to receive unilateral VATS LVRS performed by either the awake nonresectional method in 32 patients or by the non-awake resectional method in 31 patients. Comparative assessment of results between awake and non-awake groups have shown that 1 h after surgery, oxygenation as expressed by the ratio of arterial oxygen tension to fraction of inspired oxygen as well as arterial carbon dioxide tension, were significantly better in the awake group. Mortality and morbidity rates were 0 vs. 3.2% and 22% vs. 52% (P<0.01); median hospital stay was 6 vs. 7.5 days (P<0.04) with 21 vs. 10 patients discharged within 6 days (P=0.01). Moreover, at 6 months, FEV1, which was the clinical primary outcome measure, improved significantly in both study groups (0.28 vs. 0.29 L) with no intergroup difference. In addition in both groups, improvements in FEV1, 6MWT, FVC, RV and physical functioning quality of life measure, lasted more than 24 months. At 36 months, both freedom from contralateral treatment (55% vs. 50%; P=0.5) and survival (81% vs. 87%; P=0.5) where similar between study groups (13).
Redo LVRS
A particular poor risk sub-cohort that can meaningfully benefit by avoidance of intubated anesthesia is that entailing emphysematous patients who have lost the benefits achieved by a successful LVRS and who develop new target areas in the lung that are amenable of reoperation. In these patients the postoperative functional deterioration usually progresses along several years. As a result, redo-LVRS remains in most of instances the only therapeutic choice since many of these patients are older than 65 years of age and cannot be included in a lung transplantation waiting list.
In a 17 patients series on redo LVRS entailing completion lobectomy in seven patients and intubated resectional or awake nonresectional redo LVRS in five patients each, the mean age was 66 years whereas interval time between the first LVRS procedure and the reoperation was of 55 months. The 90-day operative mortality was 12% and included two patients who underwent one completion lobectomy and one nonanatomic lung resection under non-awake anesthesia.
The mean hospital stay was 9 days and significant improvements lasting for up to 12 months occurred in FEV1 (P<0.001), FVC (P<0.002), RV (P<0.001), 6MWT (P<0.001), and dyspnea index (P<0.001). Six months after surgery, 11 patients had an FEV1 improvement of 200 mL or more (46).
Conclusions
LVRS, has been shown to represent a highly effective treatment modality for properly selected patients with severe emphysema although in recent years it remains unexpectedly underused. Decker and co-workers (23) have reported that amongst the Society of Thoracic Surgeons database only 528 patients underwent non-awake LVRS during an 8.5-year period. This figure has led them to highlight the need to invest in future analyses to identify determinants of adjusted surgery-specific quality assessments.
What is arguable is that the Achilles heel of resectional LVRS is not related to doubts on its efficacy but rather to fears of the significant perioperative morbidity that has been associated with this treatment modality and that can meaningfully increase health care costs.
Within the framework of available investigational methods, the awake nonresectional LVRS method, which does not entail use of any expensive device, avoids removal of lung tissue and can be quickly performed in spontaneously ventilating awake subjects, has shown promise in uni-center studies and awaits now to be tested through well designed, multi-institutional controlled trials.
Having matured an experience with several types of thoracic surgery procedures performed through awake anesthesia we can affirm that candidate to LVRS due to emphysema are amongst the patients who can benefit most from an awake anesthesia management. In fact, we have found that perioperative breathing pattern, oxygenation promptness of resumption of daily-life activities, and hospital stay are dramatically better in patients undergoing awake LVRS if compared with those of patients operated on by general anesthesia with single-lung ventilation.
In conclusion, the fear of performing awake LVRS in delicate subjects with severe emphysema and poor pulmonary function is fully understandable as nicely underlined during the discussion about a paper on awake LVRS presented at an international meeting, when one of the moderators affirmed: “It is a little intimidating for some of us to think about having one of these critically ill patients be wide awake while we make holes in their chest and operate on them” (12).
Nonetheless, experience with nonintubated and awake thoracic surgery is increasing worldwide and it is possible that in the near future, a number of thoracic surgeons who will have gained confidence with both non-awake and awake LVRS will rather consider somewhat more intimidating performing LVRS in intubated, non-awake patients.
Acknowledgements
We thank Miss Aurora Pompeo for accomplishment of the ATSRG picture.
Disclosure: The authors declare no conflict of interest.
References
- American Lung Association. Trends in COPD (emphysema and chronic bronchitis): morbidity and mortality. February 2010. Accessed on Dec 30th, 2010. Available online: http://www.lungusa.org/finding-cures/our-research/trend-reports/copd-trend-report.pdf
- Global initiative for chronic Obstructive Lung disease. Global Strategy for Diagnosis, Management, and Prevention of COPD. Bethesda (MD): GOLD, 2013. Accessed on October 30th, 2013. Available online: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [PubMed]
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [PubMed]
- Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106-16; discussion 116-9. [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [PubMed]
- Ingenito EP, Reilly JJ, Mentzer SJ, et al. Bronchoscopic volume reduction: a safe and effective alternative to surgical therapy for emphysema. Am J Respir Crit Care Med 2001;164:295-301. [PubMed]
- Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet 2011;378:997-1005. [PubMed]
- Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574-82. [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surger. Ann Surg 2006;243:131-6. [PubMed]
- Pompeo E, Mineo TC. Two-year improvement in multidimensional body mass index, airflow obstruction, dyspnea, and exercise capacity index after nonresectional lung volume reduction surgery in awake patients. Ann Thorac Surg 2007;84:1862-9; discussion 1862-9.
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Brantigan OC, Mueller E. Surgical treatment of pulmonary emphysema. Am Surg 1957;23:789-804. [PubMed]
- Brantigan OC, Mueller E, Kress MB. A surgical approach to pulmonary emphysema. Am Rev Respir Dis 1959;80:194-206. [PubMed]
- Keenan RJ, Landreneau RJ, Sciurba FC, et al. Unilateral thoracoscopic surgical approach for diffuse emphysema. J Thorac Cardiovasc Surg 1996;111:308-15; discussion 315-6. [PubMed]
- Mineo TC, Pompeo E, Simonetti G, et al. Unilateral thoracoscopic reduction pneumoplasty for asymmetric emphysema. Eur J Cardiothorac Surg 1998;14:33-9. [PubMed]
- Kotloff RM, Tino G, Palevsky HI, et al. Comparison of short-term functional outcomes following unilateral and bilateral lung volume reduction surgery. Chest 1998;113:890-5. [PubMed]
- Bingisser R, Zollinger A, Hauser M, et al. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996;112:875-82. [PubMed]
- Pompeo E, Marino M, Nofroni I, et al. Reduction pneumoplasty versus respiratory rehabilitation in severe emphysema: a randomized study. Pulmonary Emphysema Research Group. Ann Thorac Surg 2000;70:948-53; discussion 954. [PubMed]
- Gelb AF, McKenna RJ Jr, Brenner M, et al. Lung function 5 yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med 2001;163:1562-6. [PubMed]
- Ramsey SD, Berry K, Etzioni R, et al. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med. 2003;348:2092-102. [PubMed]
- Decker MR, Leverson GE, Jaoude WA, et al. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg 2014;148:2651-8.e1.
- Brantigan OC, Kress MB, Mueller EA. The surgical approach to pulmonary emphysema. 1961. Chest 2009;136:e30. [PubMed]
- Crosa-Dorado VL, Pomi J, Perez-Penco EJ. Treatment of dyspnea in emphysema pulmonary remodeling: hemo and pneumostatic suturing of the emphysematous lung. Res Surg 1992;4:1-4.
- Swanson SJ, Mentzer SJ, DeCamp MM Jr, et al. No-cut thoracoscopic lung plication: a new technique for lung volume reduction surgery. J Am Coll Surg 1997;185:25-32. [PubMed]
- Iwasaki M, Nishiumi N, Kaga K, et al. Application of the fold plication method for unilateral lung volume reduction in pulmonary emphysema. Ann Thorac Surg 1999;67:815-7. [PubMed]
- Pompeo E. Lung volume reduction surgery for emphysema treatment: state of the art and perspectives. ISRN Pulmonology, 2014. Available online: http://dx.doi.org/10.1155/2014/418092
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [PubMed]
- Piccioni F, Langer M, Fumagalli L, et al. Thoracic paravertebral anaesthesia for awake video-assisted thoracoscopic surgery daily. Anaesthesia 2010;65:1221-4. [PubMed]
- McKenna RJ Jr, Brenner M, Fischel RJ, et al. Should lung volume reduction for emphysema be unilateral or bilateral? J Thorac Cardiovasc Surg 1996;112:1331-8; discussion 1338-9. [PubMed]
- Argenziano M, Thomashow B, Jellen PA, et al. Functional comparison of unilateral versus bilateral lung volume reduction surgery. Ann Thorac Surg 1997;64:321-6; discussion 326-7. [PubMed]
- Pompeo E, Sergiacomi G, Nofroni I, et al. Morphologic grading of emphysema is useful in the selection of candidates for unilateral or bilateral reduction pneumoplasty. Eur J Cardiothorac Surg 2000;17:680-6. [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Results of unilateral lung volume reduction surgery in patients with distinct heterogeneity of emphysema between lungs. J Thorac Cardiovasc Surg 2005;129:73-9. [PubMed]
- Brenner M, McKenna RJ Jr, Gelb AF, et al. Rate of FEV1 change following lung volume reduction surgery. Chest 1998;113:652-9. [PubMed]
- Pompeo E, Mineo TC; Pulmonary Emphysema Research group. Long-term outcome of staged versus one-stage bilateral thoracoscopic reduction pneumoplasty. Eur J Cardiothorac Surg 2002;21:627-33; discussion 633. [PubMed]
- Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg 2009;35:822-8; discussion 828. [PubMed]
- Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006;82:431-43. [PubMed]
- Cremona G, Barberà JA, Melgosa T, et al. Mechanisms of gas exchange response to lung volume reduction surgery in severe emphysema. J Appl Physiol (1985) 2011;110:1036-45. [PubMed]
- Mineo TC, Ambrogi V, Pompeo E, et al. Body weight and nutritional changes after reduction pneumoplasty for severe emphysema: a randomized study. J Thorac Cardiovasc Surg 2002;124:660-7. [PubMed]
- Mineo TC, Pompeo E, Rogliani P, et al. Effect of lung volume reduction surgery for severe emphysema on right ventricular function. Am J Respir Crit Care Med 2002;165:489-94. [PubMed]
- Jörgensen K, Houltz E, Westfelt U, et al. Effects of lung volume reduction surgery on left ventricular diastolic filling and dimensions in patients with severe emphysema. Chest 2003;124:1863-70. [PubMed]
- Kozora E, Emery CF, Ellison MC, et al. Improved neurobehavioral functioning in emphysema patients following lung volume reduction surgery compared with medical therapy. Chest 2005;128:2653-63. [PubMed]
- Homan S, Porter S, Peacock M, et al. Increased effective lung volume following lung volume reduction surgery in emphysema. Chest 2001;120:1157-62. [PubMed]
- Bloch KE, Li Y, Zhang J, et al. Effect of surgical lung volume reduction on breathing patterns in severe pulmonary emphysema. Am J Respir Crit Care Med 1997;156:553-60. [PubMed]
- Tacconi F, Pompeo E, Forcella D, et al. Lung volume reduction reoperations. Ann Thorac Surg 2008;85:1171-7. [PubMed]


