Non-intubated video-assisted thoracic surgery as the modality of choice for treatment of recurrent pleural effusions
Authors’ introduction:
Figure 1 is a picture of the two authors from the Sinai Hospital of Baltimore in Baltimore, USA. They are Dr. Solange E. Cox (right), a Surgery Resident at Sinai Hospital, and Dr. Mark R. Katlic (left), who is the Chairman of Department of Surgery.
Introduction
Pleural effusions are a common manifestation of various types of malignant disease and the presence of a pleural effusion often portends a poor prognosis. Pleural effusions are associated with a significant amount of morbidity and mortality making their management crucial for establishing a better quality of life for the patients with symptomatic complications. There are several means of managing pleural effusions which include thoracostomy tube placement with bedside chemical pleurodesis, thoracentesis, placement of an indwelling pleural catheter, pleurectomy and video-assisted thoracic surgery (VATS) drainage with chemical talc pleurodesis (1). The treatment modality chosen is commonly based on the medical stability and needs of the patient. Malignant pleural effusions will often re-accumulate following initial drainage which makes effective pleurodesis to prevent recurrence, an important factor in their definitive management. Ideally the best management of recurrent pleural effusions should provide complete initial drainage, adequate pleurodesis to prevent re-accumulation and have a high diagnostic yield for biopsy in the event that a tissue sample is needed to establish a definitive diagnosis and treatment plan (2).
Treatment methods for recurrent pleural effusions
VATS with talc pleurodesis
VATS has become a more common method for treating recurrent pleural effusions over the past few years. It is a less invasive alternative to a thoracotomy with or without pleurectomy. It provides various advantages over other methods of treatment which include complete initial drainage under direct visualization, talc placement for pleurodesis under direct visualization which allows for more adequate placement of talc and subsequently more effective pleurodesis. In addition, this procedure is minimally invasive and can be done using local anesthetic and intravenous sedation thus avoiding the risks associated with intubation and resulting in the highest patient satisfaction rate of all treatment modalities (3-7). One of the most important advantages of this treatment method is the ability to obtain a tissue sample for diagnosis under direct visualization which increases the diagnostic yield of the biopsy (8). The disadvantages of this method include the higher cost associated with use of the operating room and increased required resources. This treatment method may also require a short inpatient stay, however with the use of local anesthetic and IV sedation this procedure can be done as an outpatient if the patient is an appropriate candidate. Patient undergoing this procedure often times also require chest tube placement for continued drainage initially. The patients often go home with the chest tube which allows for the procedure to be done on an outpatient basis. The rate of pleurodesis with this procedure is high and one of the most successful of any treatment modality. Thus, despite the fact that the patient will go home with a thoracostomy tube initially, this tube is usually not needed over the long term. Elective patients are discharged the same or next day, usually with a small drainage container (Atrium Mini-Express®, Atrium Medical Corporation, Hudson, New Hampshire, USA) attached to the chest tube or, in some cases, with a PleurX® catheter (CareFusion Corporation, San Diego, CA, USA). The chest tube is removed in the office as appropriate.
Thoracostomy tube and talc slurry
Tube thoracostomy is a minimally invasive bedside procedure with a low associated cost. It is well tolerated in patients who are not appropriate candidates for VATS procedures. It is an outpatient procedure and talc slurry can be instilled through the tube to achieve pleurodesis (9,10). Despite the low cost and general ease of placement, the tube does not allow for direct visualization of the pleural cavity. In some instances complete drainage may not initially be obtained. The lack of direct visualization makes placement of the talc for chemical pleurodesis not as optimal leading to lower rates of pleurodesis than with VATS procedures where the talc can be placed under direct visualization (11). With thoracostomy tubes there may be a need for additional procedures for drainage if adequate pleurodesis is not achieved. Patient satisfaction is also lower for bedside tube placement as this is associated with a higher level of anxiety for the fully awake patient and a higher amount of pain since this procedure is not done with IV sedation but instead only using local anesthetic. Additionally patients often go home with a chest tube in place. Based on the fact that a lower rate of pleurodesis is achieved with this procedure the chest tube may remain in place for longer periods than with a VATS procedure with IV sedation and local anesthetic.
Thoracentesis
This is a quick cost effective method of treating pleural effusions. It is a minimally invasive outpatient procedure with minimal risk of complications (12,13). Thoracentesis has a less important role in the setting of a recurrent pleural effusion due to the fact that this procedure does not involve any aspect of preventing recurrence. There is no pleurodesis associated with this procedure and considering that most of these effusions will re-accumulate this method is not a definitive treatment method (14). In addition to this, the diagnostic yield for tissue diagnosis is low as a tissue biopsy is typically not obtained with this procedure.
Long term indwelling pleural catheter
Placement of a long term indwelling pleural PleurX® catheter (CareFusion Corporation, San Diego, CA, USA), is a generally well tolerated procedure (9,10,15). It is a minimally invasive, outpatient, cost effective method of treatment for pleural effusions and allows for more patient control over the drainage of the effusions (9,15). The indwelling catheter is associated however with a higher degree of complications including clogging of the catheter, increased risk of infection, and pain at the catheter site (14,15). All of these may create the need for additional procedures. In addition to this, pleural catheters do not facilitate the instillation of any form of chemical for pleurodesis (14,16). Also this method does not allow for obtaining any tissue for diagnosis. Most recurrent pleural effusions are the result of a malignant process, and thus tissue diagnosis if often needed to direct future medical management making this method less ideal for patients.
Discussion/conclusions
There are currently various options for treatment and management of recurrent pleural effusions (Table 1). The goals of treatment include methods which involve the least number of repeated procedures, the best patient satisfaction, least amount of pain and anxiety inflicted on the patient, lowest length of stay in the hospital, cost effective, preferably an outpatient procedure and optimally a method which has a high diagnostic yield for tissue diagnosis and a high rate of pleurodesis. Of the various methods described the most ideal in achieving the goals of treatment is non-intubated VATS with talc pleurodesis with local anesthetic and intravenous sedation. VATS with talc pleurodesis is by far the best option for patients and the use of intravenous sedation and local anesthetic reduce the overall cost, length of stay and potential complications associated with the use of intubation and general anesthesia. In patients who undergo the VATS procedure after local and intravenous sedation the average hospital stay is 0-1 days whereas the same procedure with intubated patients has a hospital stay of 6 days on average. Unlike thoracentesis, pleural catheter placements and tube thoracostomy this method allows for direct visualization of the pleural cavity. It also has the highest diagnostic yield for tissue diagnosis. There is a significantly high risk of morbidity and mortality associated with more invasive methods such as a thoracotomy or a pleurectomy (17) than with a VATS with talc pleurodesis. Given the many overall benefits of non-intubated VATS with talc pleurodesis for recurrent pleural effusions, this method stands out as the best treatment modality currently available to patients. Evidence suggests that this procedure should be the standard of care for all appropriate candidates (Table 2).
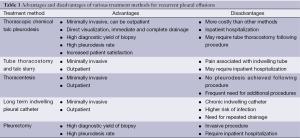
Full table
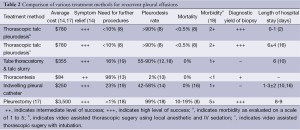
Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Bethune N. Pleural poudrage: a new technique for the deliberate production of pleural adhesion as a preliminary to lobectomy. J Thorac Surg 1935;4:251-61.
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [PubMed]
- Rusch VW, Mountain C. Thoracoscopy under regional anesthesia for the diagnosis and management of pleural disease. Am J Surg 1987;154:274-8. [PubMed]
- Pompeo E. Awake thoracic surgery--is it worth the trouble? Semin Thorac Cardiovasc Surg 2012;24:106-14. [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii54-60. [PubMed]
- Migliore M, Giuliano R, Aziz T, et al. Four-step local anesthesia and sedation for thoracoscopic diagnosis and management of pleural diseases. Chest 2002;121:2032-5. [PubMed]
- Danby CA, Adebonojo SA, Moritz DM. Video-assisted talc pleurodesis for malignant pleural effusions utilizing local anesthesia and I.V. sedation. Chest 1998;113:739-42. [PubMed]
- Harris RJ, Kavuru MS, Rice TW, et al. The diagnostic and therapeutic utility of thoracoscopy: a review. Chest 1995;108:828-41. [PubMed]
- Olden AM, Holloway R. Treatment of malignant pleural effusion: PleuRx catheter or talc pleurodesis? A cost-effectiveness analysis. J Palliat Med 2010;13:59-65. [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [PubMed]
- Stefani A, Natali P, Casali C, et al. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg 2006;30:827-32. [PubMed]
- Muduly D, Deo S, Subi Ts, et al. An Update in the Management of Malignant Pleural Effusion. Indian J Palliat Care 2011;17:98-103. [PubMed]
- Thai V, Damant R. Malignant pleural effusions: interventional management #157. J Palliat Med 2009;12:1051-2. [PubMed]
- Haas AR, Sterman DH, Musani AI. Malignant Pleural Effusions*: Management Options With Consideration of Coding, Billing, and a Decision Approach. Chest 2007;132:1036-41. [PubMed]
- Penz ED, Mishra EK, Davies HE, et al. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest 2014;146:991-1000. [PubMed]
- Freeman RK, Ascioti AJ, Mahidhara RS. A propensity-matched comparison of pleurodesis or tunneled pleural catheter in patients undergoing diagnostic thoracoscopy for malignancy. Ann Thorac Surg 2013;96:259-63: discussion 263-4.
- Fry WA, Khandekar JD. Parietal pleurectomy for malignant pleural effusion. Ann Surg Oncol 1995;2:160-4. [PubMed]
- Austin EH, Flye MW. The treatment of recurrent malignant pleural effusion. Ann Thorac Surg 1979;28:190-203. [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [PubMed]



