Roscovitine in cancer and other diseases
Introduction
Protein phosphorylation by protein kinases plays a central role in the regulation of various cell processes, such as proliferation, cell cycle, differentiation and apoptosis. Because of that, deregulation of kinase activity can result in remarkable changes of these processes. Deregulated kinases are often found to be oncogenic and can be central for the survival and spread of cancer cells (1). Likewise, the phosphorylation of some proteins, such as AKT (2-4), EGFR (5,6), ERBB2 (5,7-9), SCH1 (10) and RB1 (11) is associated with prognosis in cancers.
Cyclin-dependent kinases (CDK) are protein kinases of CMGC group of kinases, which play an essential role in the control of the cell cycle and/or proliferation and transcription. There are 21 CDK genes in human genome, 11 of which are so called “classical” CDKs. These CDKs are responsible for the activation of the cell cycle of quiescent cells as well as for the from G1/M and G2/S transitions of the cell cycle. Different CDKs are involved in different checkpoints of the cells cycle. CDK4 and 6 initiate the transition from quiescence to proliferation; CDK2 coordinates cell progression from G1 through S-phase, while CDK1 is a universal M-phase promoting factor. These CDKs also associate with different cyclins: CDK4/6 with cyclin D, CDK2 first with cyclin E and then with cyclin A and CDK1 with cyclin B (12). Increased levels of CDK4, CDK6 and CDK2 activities have been observed in many different cancers. Overexpression of CDK activators such as cyclin D1, cyclin E and cyclin A and the activating phosphatases CDC25A and CDC25B or loss of function of the CDK inhibitors CDKN2A, CDKN1A and CDKN1B are major causes for the overactivation of CDKs (13). The fundamental role of CDKs in the cell cycle and proliferation, and their well-recognized role in the pathology of cancer make them attractive drug targets. Therefore, the search for the inhibitors of CDKs has been one of the interests of both academic and industrial scientific communities. To date, more than 30 different CDK small molecule inhibitors are developed: broad-range inhibitors (such as flavopiridol, olomoucine, roscovitine, kenpaullone, SNS-032, AT7519, AG-024322, R547), specific inhibitors (such as fascaplysin, ryuvidine, purvalanol A, NU2058, BML-259, SU 9516, PD 0332991, P-276–00) and third generation inhibitors (such as CR8#13, dinaciclib). Many of these inhibitors had entered different stages of clinical trials (14-18).
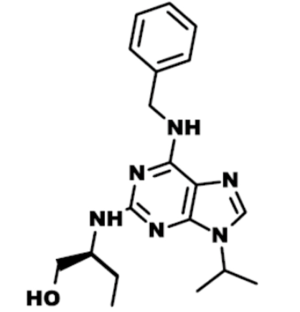
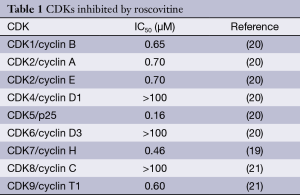
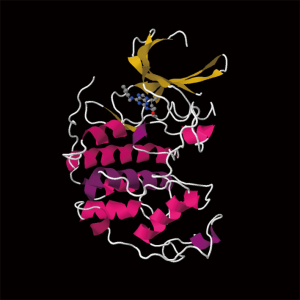
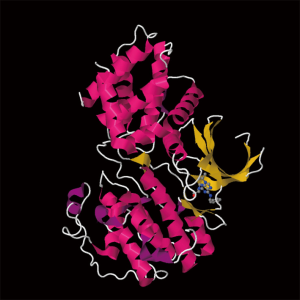
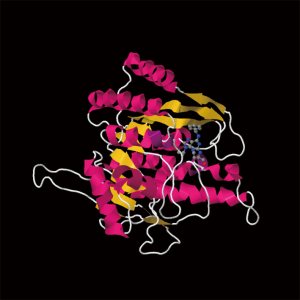
Roscovitine (named after Roscoff—a French town, where the lab which discovered the compound was located) (Figure 1) is also known as (R)-Roscovitine, CY-202, and Seliciclib. The systematic name of roscovitine is (2R)-2-{[6-(Benzylamino)-9-isopropyl-9H-purin-2-yl] amino}-1-butanol, chemical formula C19H26N6O, molecular weight—354.45. It is a white powder that is soluble in DMSO (up to 50 mM) and in 50 mM HCl with the pH adjusted to 2.5. Roscovitine belongs to the family of purines, which all share the basic ring structure and include biologically important molecules such as ATP, cyclic AMP, NAD, FAD, adenine and guanine, etc. It acts by competing with ATP for binding at the ATP-binding site of CDKs by interacting with the amino acids that line up the ATP-binding pocket of the CDK catalytic domain. In case of CDK2, the interaction mostly consists of two hydrogen bonds (involving N7 and N6 of the purine) with backbone atoms of Leu83. A weak hydrogen bond is also formed between O1 and a water molecule and the benzyl ring of roscovitine is facing the outside of the ATP-binding pocket. This interaction prevents ATP from binding the kinase, and thus catalytic reaction cannot be performed (19). Roscovitine is a broad-range purine analog inhibitor, which inhibits CDK1, CDK2, CDK5, CDK7and CDK9 (IC50 ~0.2-0.7 µM) but is a poor inhibitor for CDK4, CDK6 and CDK8 (IC50 >100 µM) (Table 1). Only several kinases are sensitive to roscovitine in the 1-40 µM range (CaMK2, CK1α, CK1δ, DYRK1A, EPHB2, ERK1, ERK2, FAK, and IRAK4), but other kinases are insensitive to roscovitine (20,22,23). It has been shown that roscovitine acts by competing with ATP for binding at the ATP-binding site of CDKs. This binding at the catalytic site was confirmed by direct cocrystallization of roscovitine with CDK2 (19) (Figure 2) and CDK5/p25 (24) (Figure 3). Affinity chromatography with sepharose-immobilized roscovitine shown that roscovitine interacts with PDXK from all species that have been investigated (22). Roscovitine has been cocrystallized with sheep pyridoxal kinase (PDXK) (25) (Figure 4) and the interaction investigated in depth. Unexpectedly, roscovitine was located in the pyridoxal-binding site, instead of the ATP-binding site and the atoms of roscovitine involved in the binding to PDXK were different from those involved in the binding to CDKs. The roscovitine/CDK cocrystal structures bred a lot of interest and significantly stimulated the search for new CDK inhibitors or the rational optimization of the existing ones. To date, more than 20 inhibitors have been cocrystallized with CDKs. Roscovitine is a widely used inhibitor in both basic research and disease research. It has been tested in several phase I and II clinical trials both as monotherapy and combination therapy in several human cancers.
Full table
Cellular effects and preclinical tests
The effects of roscovitine had been evaluated on a wide variety of cancer cell lines.
Roughly, two main processes have been observed: cell cycle arrest and an initiation of apoptosis. Roscovitine arrests the cell cycle in all tested cancer cell lines with the average IC50 value of about 15 µM (19,26). Roscovitine blocks cell cycle in G0, G1, S or G2/M, depending on the dose, time or cell line, by directly inhibiting CDKs. Moreover, there are many molecular processes that depend on the activity of CDKs activity, and thus, are inhibited by roscovitine. Those include pathways such as Ras-MAPK (27), NF-κB (28), p53 (28,29), estrogen receptor (30), JAK-STAT (31), etc.
Roscovitine induces apoptosis in many cancer cell lines at all phases of the cell cycle (32). It has been shown to downregulate Bcl-2 (33), Mcl-1 (34), survivin (35) and XIAP (35) and upregulate p53 (36), p53AIP1 (37) and Bcl-x (38) and others (39).
In addition, roscovitine has been shown to have a synergistic effect with other anti-cancer agents, such as doxorubicin (40,41), taxol (41), 5-fluorouracil (41), vinblastine (41), alemtuzumab (42), paclitaxel (43), trastuzumab (44), cisplatin (45), radiation (46), irinotecan (47), etoposide (48) and tamoxifen (49).
The anti-cancer action of roscovitine has also been investigated in various cancer xenografts. Xenograft experiment using LoVo human colorectal cancer cells grafted into CD1 nude mice showed a significant antitumor effect of roscovitine (100 mg/kg administered intraperitonealy, 3 times daily for 5 days) with a 45% reduction in tumor growth compared to control animals (32). Same publication shows xenograft experiment using MESSA-DX5 human uterine carcinoma cells grafted into CD1 nude mice. Roscovitine (orally administered, 500 mg/kg 3 times daily for 4 days) displayed a significant reduction in tumor growth (62%). MDA-MB 231 tumor xenografts in nude mice increased growth inhibition of 73% combined treatment group (100 mg/kg roscovitine + 7.5 Gy irradiation) as compared with 54% for the irradiation only group (P=0.02) (46). Roscovitine (administered orally 500 mg/kg) caused a 79% reduction in the tumor growth compared to controls at day 5 (P<0.05) in the HCT116 human colon cancer xenograft model in nude mice (50). Mice xenografts of A4573 Ewing’s sarcoma cells treated with roscovitine (administered intraperitonealy 50 mg/kg, for 5 days) had grown only ~1.25-fold relative to their size at the time of treatment start, while tumors in untreated mice had already reached ~14.5-fold their original size (51). Roscovitine also showed 35% tumor growth inhibition in a PC-3 prostate cancer xenograft model (52). Osteosarcoma tumor xenograft in B6D2F1 mice weight was reduced by 55% in the animals receiving roscovitine (administered orally 300 mg/kg a day) during resting time and by 35% in those treated at active time compared with untreated controls (P<0.001) (53). HT29 human colon cancer xenografts in nude mice had a 68% and 80% tumor reduction using 10 and 40 mg/kg roscovitine respectively, administered intraperitoneally (P<0.001) (47). MCF7 breast cancer xenografts in nude mice showed statistically significant inhibition of tumor growth of 48% and 70%, when treated single agent (orally administered 1.5 mg/kg doxorubicin or 400 mg/kg roscovitine, twice a day) compared with seliciclib + doxorubicin, respectively relative to the vehicle control group (P<0.05) (54). Immunodeficient mice, subcutaneously injected with RAMOS or HBL-2 cell lines, treated with roscovitine (12.5 mg/mouse) and TRAIL (250 µg/mouse), as single agents or in combination, showed that significantly suppressed the growth of both RAMOS (P=0.0221) and HBL-2 (P=0.0014) tumors compared to single-agent approaches (55). Xenograft study, using hormone therapy-resistant breast cancer cell lines (MCF-7-TamR, MCF-7-LTLTca and MCF-7-HER2), treated with roscovitine (administered orally at a dose of 100 mg/kg), showed significantly smaller tumor volumes and smaller tumor sizes (P<0.05) (56). DNA synthesis was inhibited 75% by 50 µM roscovitine treatment in tissue mini-units were prepared from tumor specimens obtained from the rat G2 glioma model (57). The preclinical studies, described in this section, are summarized in Table 2.
Preclinical studies of roscovitine in other pathologies
Cancers, however, are not the only diseases, that have been investigated in animal models. Apparently, CDKs are involved in quite a few disorders, and thus CDK inhibition by roscovitine can help to overcome them. One of the most investigated groups of diseases in animal models is kidney diseases. Polycystic kidney disease is one of those, and roscovitine produces effective arrest of it in jck and cpk mouse models of polycystic kidney disease (75). In a Pkd1 conditional knockout mouse model, which results in a rapid onset polycystic kidney disease at day 5, roscovitine-treated group (administered 100 mg/kg, once a day) showed a significant inhibition of polycystic kidney disease, revealed by a decrease in kidney to body weight ratio, cystic volume and blood urea nitrogen (P<0.05) (76). Glomerulonephritis is another highly investigated disease, or rather an outcome of a number of diseases. In mice with systemic lupus, roscovitine treatment (100 or 200 mg/kg), combined with methylprednisolone extended the survival (71% and 77% of those treated with 100 and 200 mg/kg, respectively, as compared with 31% of control), limited proteinuria (43% in the group receiving 100 mg/kg of roscovitine and 23% in the group receiving 200 mg/kg of roscovitine, compared with 85% in control) and renal damage and reduced immunologic signs of disease more than treatment with any of the compound separately (79). There was a 30% decrease in the urine protein/creatinine ratio at day 10 in rats with Thy1 glomerulonephritis given roscovitine compared to control (P<0.05) (62). Mesangial cell proliferation was reduced by >50% at days 5 and 10 in the Roscovitine prevention group, and at day 5 in the treatment group (P<0.0001). Similar study showed, that roscovitine treatment in rats with Thy1 glomerulonephritis preserved renal function, such as increased creatinine clearance (P<0.007), reduced proteinuria (P<0.02), increased urinary excretion (P<0.02), reduced haematuria (P<0.02) (63). In the study of rats with passive Heymann nephritis, compared to control group, treatment with low-dose roscovitine (25 mg/kg/day) decreased the number of glomerular mitotic figures at day 30 by 22% and by 61% in the high-dose group (50 mg/kg/day) (66). Cell proliferation was significantly lower in the high-dose roscovitine-treated group as compared to the vehicle-only–treated group (P<0.05). Rat model of ischemia–reperfusion injury showed protective effect of roscovitine: there was noticeable acute tubular necrosis in control animals, but roscovitine-treated group showed negligible histologic signs of ischemic injury (68). The roscovitine-treated group showed lower values of both blood urea nitrogen and creatinine than control group (P<0.05).
Roscovitine was also investigated in pain treatment in animal models. Bone cancer mice models were used to investigate whether roscovitine could attenuate bone cancer pain. At day 14 after inoculation, osteosarcoma significantly enhanced mechanical allodynia and thermal hyperalgesia, which was reduced by roscovitine (intrathecal administration 5, 10 or 20 µg) by downregulation of the expression of NR2A (72). Downregulation of the expression of NR2A is also involved in pain alleviation by roscovitine in a rat model of chronic compression of dorsal root ganglion (73). Roscovitine (intrathecal administration 25, 50 or 100 µg) reduced thermal hyperalgesia (P<0.05) and mechanical allodynia (P<0.05) induced by intraoperative remifentanil administration (74).
Neurodegeneration and retinal degeneration is another well studied field with regard to roscovitine. In rabbit glaucoma model, instillation roscovitine significantly lowered the increased intraocular pressure, amplified the effects of tunicamycin and increased oxygen-glucose deprivation-induced cell death (61). Roscovitine decreased apoptosis of retinal photoreceptor cells in Rd1 retinal degeneration mouse model (P<0.01) (77). Pretreatment with roscovitine at 20 µmol/L for 24 h was protective against HIV protein gp120 toxicity in an animal model of HIV-protein mediated neurotoxicity (P<0.05) (70).
Roscovitine was also shown to reduce lung inflammation (67), protect against acute graft versus host disease (64), prevent radiation-induced salivary gland dysfunction (78) and had somewhat decreased Herpetic keratitis (65). Roscovitine was also shown to inhibit the replication of herpes simplex virus and HIV1 by targeting cellular proteins (21). The preclinical studies, described in this section, are summarized in Table 2.
Full table
Clinical trials
A phase I clinical trial with roscovitine showed no objective tumor responses, but disease stabilization was observed in 38% patients (8/21) (59). Patients were treated with doses of 100, 200 and 800 mg twice daily. Dose-limiting toxicities were seen at 800 mg and included fatigue, skin rash, hyponatremia and hypokalemia. Emesis and reversible abnormal liver function were also observed. Another phase I clinical trial enrolled 56 patients, which were treated according to three schedules: schedule A consisted of 5 consecutive days every 3 weeks, schedule B of 10 consecutive days followed by 2 weeks off and schedule C of 3 consecutive days every 2 weeks. In schedule A, the dose of 1,600 mg two times daily was considered intolerable, causing asthenia, nausea, vomiting and hypokalaemia. In schedule B, 800 mg two times daily was considered intolerable, causing hypokalaemia. In schedule C, 1,800 mg two times daily are considered intolerable, causing hypokalaemia. One patient with hepatocellular carcinoma showed partial response (800 mg bid, schedule B) and six patients achieved tumor stabilization: two patients with non-small cell lung cancer (1,600 mg, schedule A, and 1,800 mg, schedule C), one with parotid cylindroma (100 mg, schedule A), one with corticosurrenaloma (1,000 mg, schedule A), one with thymic carcinoma (1,000 mg, schedule A) and one with adenocarcinoma of unknown primary (400 mg, schedule A) (58).
Twenty-three nasopharyngeal carcinoma patients were enrolled in phase II roscovitine clinical trial. Dose limiting toxicities were observed in 4 patients, common adverse events included fatigue, nausea, vomiting, constipation, cough, fever, hypokalemia, hyponatremia, and elevation in aspartate transaminase/ alanine transaminase, most of which were mild or moderate (69). Another phase II study of roscovitine as a single agent in patients with previously-treated non-small cell lung cancer has been closed with no data reported (71). Four phase II studies were announced, however no data is published up to date. One of them was a monotherapy trial in haematological B-cell malignancies, while the other three are combination trials with gemcitabine/cisplatin in first-line non-small cell lung cancer, with docetaxel in second line non-small cell lung cancer and with capecitabine in metastatic breast cancer (60). The clinical studies, described in this section, are summarized in Table 2.
The future of roscovitine and its derivatives in cancer and other diseases
Despite many successful preclinical studies with roscovitine, clinical trials are not very encouraging. It would seem that combination therapies with roscovitine could be more promising than monotherapy, thus more chemotherapeutic agents as well as other targeted drugs should be evaluated in combination.
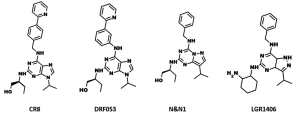
Another hope lies with next generation derivatives of roscovitine. Roscovitine derivative CR8 (Figure 5) was also shown to inhibit renal cystogenesis in Pkd1-conditional knockout in above mentioned study (76). It was shown to provide neuroprotection in experimental traumatic brain injury (80). CDK/CK1 dual-specificity inhibitors (Figure 5), derived from roscovitine, were shown to inhibit cell proliferation and prevent the production of amyloid-beta and may have applications in Alzheimer's disease and cancers (81). N-&-N (Figure 5), another class of roscovitine derivatives, showed improved anticancer properties and caused apoptosis in a panel of different cell lines (82). In addition, these compounds have reduced affinity for Erk2 and pyridoxal kinase. The roscovitine derivative LGR1406 (Figure 5) showed much more potent (IC50 =3.0 µM) antiproliferative activity than roscovitine (IC50 =16.9 µM), halting vascular smooth muscle cells (83). Recently, several CDK inhibitors, related to roscovitine (Figure 6), were reported as anti-malarial agents (84). The roscovitine derivatives, described in this section, are summarized in Table 3.

Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hunter T, Cooper JA. Protein-tyrosine kinases. Annu Rev Biochem 1985;54:897-930. [PubMed]
- Cicenas J, Kueng W, Wight E, et al. Prognostic value of phosphorylated Akt in primary breast cancer. [abstract]. In: Proceedings of the 95th annual meeting of the American Association for Cancer Research (AACR). Orlando: 2004.
- Cicenas J, Urban P, Vuaroqueaux V, et al. Increased level of phosphorylated akt measured by chemiluminescence-linked immunosorbent assay is a predictor of poor prognosis in primary breast cancer overexpressing ErbB-2. Breast Cancer Res 2005;7:R394-401. [PubMed]
- Cicenas J. The potential role of Akt phosphorylation in human cancers. Int J Biol Markers 2008;23:1-9. [PubMed]
- Cicenas J. The potential role of EGFR/ErbB2 heterodimer in breast cancer. Expert opinion Ther Patents 2007;17:607-16.
- Kanematsu T, Yano S, Uehara H, et al. Phosphorylation, but not overexpression, of epidermal growth factor receptor is associated with poor prognosis of non-small cell lung cancer patients. Oncol Res 2003;13:289-98. [PubMed]
- Cicenas J, Urban P, Küng W, et al. Phosphorylation of tyrosine 1248-ERBB2 measured by chemiluminescence-linked immunoassay is an independent predictor of poor prognosis in primary breast cancer patients. Eur J Cancer 2006;42:636-45. [PubMed]
- Eppenberger-Castori S, Kueng W, Benz C, et al. Prognostic and predictive significance of ErbB-2 breast tumor levels measured by enzyme immunoassay. J Clin Oncol 2001;19:645-56. [PubMed]
- DiGiovanna MP, Stern DF, Edgerton SM, et al. Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol 2005;23:1152-60. [PubMed]
- Cicenas J, Küng W, Eppenberger U, et al. Increased level of phosphorylated ShcA measured by chemiluminescence-linked immunoassay is a predictor of good prognosis in primary breast cancer expressing low levels of estrogen receptor. Cancers 2010;2:153-64. [PubMed]
- Derenzini M, Montanaro L, Vici M, et al. Relationship between the RB1 mRNA level and the expression of phosphorylated RB protein in human breast cancers: their relevance in cell proliferation activity and patient clinical outcome. Histol Histopathol 2007;22:505-13. [PubMed]
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci 2005;30:630-41. [PubMed]
- Welburn JP, Endicott JA. Inhibition of the cell cycle with chemical inhibitors: a targeted approach. Semin Cell Dev Biol 2005;16:369-81. [PubMed]
- Cicenas J, Valius M. The CDK inhibitors in cancer research and therapy. J Cancer Res Clin Oncol 2011;137:1409-18. [PubMed]
- Cicenas J, Kalyan K, Sorokinas A, et al. Highlights of the Latest Advances in Research on CDK Inhibitors. Cancers 2014;6:2224-42. [PubMed]
- Bruyère C, Meijer L. Targeting cyclin-dependent kinases in anti-neoplastic therapy. Curr Opin Cell Biol 2013;25:772-9. [PubMed]
- Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov 2009;8:547-66. [PubMed]
- Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res 2014;20:3379-83. [PubMed]
- Azevedo WF, Leclerc S, Meijer L, et al. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem 1997;243:518-26. [PubMed]
- Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997;243:527-36. [PubMed]
- Schang LM, Bantly A, Knockaert M, et al. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J Virol 2002;76:7874-82. [PubMed]
- Bain J, McLauchlan H, Elliott M. at al. The specificities of protein kinase inhibitors: an update. Biochem J 2003;371:199-204. [PubMed]
- Bach S, Knockaert M, Lozach O, et al. Roscovitine targets: protein kinases and pyridoxal kinase. J Biol Chem 2005;280:31208-19. [PubMed]
- Mapelli M, Massimiliano L, Crovace C, et al. Mechanism of CDK5/p25 binding by CDK inhibitors. J Med Chem 2005;48:671-9. [PubMed]
- Tang L, Li MH, Cao P, et al. Crystal structure of pyridoxal kinase in complex with roscovitine and derivatives. J Biol Chem 2005;280:31220-9. [PubMed]
- Raynaud FI, Whittaker SR, Fischer PM, et al. In vitro and in vivo pharmacokinetic- pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin Cancer Res 2005;11:4875-87. [PubMed]
- Whittaker SR, Walton MI, Garrett MD, et al. The cyclin-dependent kinase inhibitor CYC202 (R-roscovitine) inhibits retinoblastoma protein phosphorylation, causes loss of Cyclin D1, and activates the mitogen-activated protein kinase pathway. Cancer Res 2004;64:262-72. [PubMed]
- Dey A, Wong ET, Cheok CF, et al. R-Roscovitine simultaneously targets both the p53 and NF-kappaB pathways and causes potentiation of apoptosis: implications in cancer therapy. Cell Death Differ 2008;15:263-73. [PubMed]
- Paprskárová M, Krystof V, Jorda R, et al. Functional p53 in cells contributes to the anticancer effect of the cyclin-dependent kinase inhibitor roscovitine. J Cell Biochem 2009;107:428-37. [PubMed]
- Węsierska-Gądek J, Gritsch D, Zulehner N, et al. Roscovitine, a selective CDK inhibitor, reduces the basal and estrogen-induced phosphorylation of ER-α in human ER-positive breast cancer cells. J Cell Biochem 2011;112:761-72. [PubMed]
- Mohapatra S, Chu B, Wei S, et al. Roscovitine inhibits STAT5 activity and induces apoptosis in the human leukemia virus type 1-transformed cell line MT-2. Cancer Res 2003;63:8523-30. [PubMed]
- McClue SJ, Blake D, Clarke R, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine). Int J Cancer 2002;102:463-8. [PubMed]
- Coker-Gurkan A, Arisan ED, Obakan P, et al. Roscovitine-treated HeLa cells finalize autophagy later than apoptosis by downregulating Bcl-2. Mol Med Rep 2015;11:1968-74. [PubMed]
- Leitch AE, Riley NA, Sheldrake TA, et al. The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur J Immunol 2010;40:1127-38. [PubMed]
- Kim EH, Kim SU, Shin DY, et al. Roscovitine sensitizes glioma cells to TRAIL-mediated apoptosis by downregulation of survivin and XIAP. Oncogene 2004;23:446-56. [PubMed]
- Wesierska-Gadek J, Wandl S, Kramer MP, et al. Roscovitine up-regulates p53 protein and induces apoptosis in human HeLaS(3) cervix carcinoma cells. J Cell Biochem 2008;105:1161-71. [PubMed]
- Wesierska-Gadek J, Gueorguieva M, Horky M. Roscovitine-induced up-regulation of p53AIP1 protein precedes the onset of apoptosis in human MCF-7 breast cancer cells. Mol Cancer Ther 2005;4:113-24. [PubMed]
- Mihara M, Shintani S, Kiyota A, et al. Cyclin-dependent kinase inhibitor (roscovitine) suppresses growth and induces apoptosis by regulating Bcl-x in head and neck squamous cell carcinoma cells. Int J Oncol 2002;21:95-101. [PubMed]
- Garrofé-Ochoa X, Cosialls AM, Ribas J, et al. Transcriptional modulation of apoptosis regulators by roscovitine and related compounds. Apoptosis 2011;16:660-70. [PubMed]
- Lambert LA, Qiao N, Hunt KK, et al. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res 2008;68:7966-74. [PubMed]
- Abaza MS, Bahman AM, Al-Attiyah RJ. Roscovitine synergizes with conventional chemo-therapeutic drugs to induce efficient apoptosis of human colorectal cancer cells. World J Gastroenterol 2008;14:5162-75. [PubMed]
- Weingrill E, Wölfler A, Strunk D, et al. Roscovitine in B-chronic lymphocytic leukemia cells: high apoptosis-inducing efficacy and synergism with alemtuzumab independent of the patients' pretreatment status Haematologica 2007;92:1286-8. [PubMed]
- Coley HM, Shotton CF, Thomas H. Seliciclib (CYC202; r-roscovitine) in combination with cytotoxic agents in human uterine sarcoma cell lines. Anticancer Res 2007;27:273-8. [PubMed]
- Fleming IN, Hogben M, Frame S, et al. Synergistic inhibition of ErbB signaling by combined treatment with seliciclib and ErbB-targeting agents. Clin Cancer Res 2008;14:4326-35. [PubMed]
- Coley HM, Shotton CF, Kokkinos MI, et al. The effects of the CDK inhibitor seliciclib alone or in combination with cisplatin in human uterine sarcoma cell lines. Gynecol Oncol 2007;105:462-9. [PubMed]
- Maggiorella L, Deutsch E, Frascogna V, et al. Enhancement of radiation response by roscovitine in human breast carcinoma in vitro and in vivo. Cancer Res 2003;63:2513-7. [PubMed]
- Abal M, Bras-Goncalves R, Judde JG, et al. Enhanced sensitivity to irinotecan by Cdk1 inhibition in the p53-deficient HT29 human colon cancer cell line. Oncogene 2004;23:1737-44. [PubMed]
- Maude SL, Enders GH. Cdk inhibition in human cells compromises chk1 function and activates a DNA damage response. Cancer Res 2005;65:780-6. [PubMed]
- Gritsch D, Maurer M, Zulehner N, et al. Tamoxifen enhances the anti-proliferative effect of roscovitine, a selective cyclin-dependent kinase inhibitor, on human ER-positive human breast cancer cells. J Exp Ther Oncol 2011;9:37-45. [PubMed]
- Raynaud FI, Whittaker SR, Fischer PM, et al. In vitro and in vivo pharmacokinetic-pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin Cancer Res 2005;11:4875-87. [PubMed]
- Tirado OM, Mateo-Lozano S, Notario V. Roscovitine is an effective inducer of apoptosis of Ewing's sarcoma family tumor cells in vitro and in vivo. Cancer Res 2005;65:9320-7. [PubMed]
- Payton M, Chung G, Yakowec P, et al. Discovery and evaluation of dual CDK1 and CDK2 inhibitors. Cancer Res 2006;66:4299-308. [PubMed]
- Iurisci I, Filipski E, Reinhardt J, et al. Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer Res 2006;66:10720-8. [PubMed]
- Appleyard MV, O'Neill MA, Murray KE, et al. Seliciclib (CYC202, R-roscovitine) enhances the antitumor effect of doxorubicin in vivo in a breast cancer xenograft model. Int J Cancer 2009;124:465-72. [PubMed]
- Molinsky J, Klanova M, Koc M, et al. Roscovitine sensitizes leukemia and lymphoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Leuk Lymphoma 2013;54:372-80. [PubMed]
- Nair BC, Vallabhaneni S, Tekmal RR, et al. Roscovitine confers tumor suppressive effect on therapy-resistant breast tumor cells. Breast Cancer Res 2011;13:R80. [PubMed]
- Yakisich JS, Vita MF, Siden A, et al. Strong inhibition of replicative DNA synthesis in the developing rat cerebral cortex and glioma cells by roscovitine. Invest New Drugs 2010;28:299-305. [PubMed]
- Le Tourneau C, Faivre S, Laurence V, et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer 2010;46:3243-50. [PubMed]
- Benson C, White J, De Bono J, et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br J Cancer 2007;96:29-37. [PubMed]
- Fischer PM, Gianella-Borradori A. Recent progress in the discovery and development of cyclin-dependent kinase inhibitors. Expert Opin Investig Drugs 2005;14:457-77. [PubMed]
- Kasai H, Imamura T, Tsuruma K, et al. Effects of roscovitine, a cell cyclin [correction of cycling]-dependent kinase inhibitor, on intraocular pressure of rabbit and retinal ganglion cell damage. Neurosci Lett 2013;535:95-9. [PubMed]
- Pippin JW, Qu Q, Meijer L, et al. Direct in vivo inhibition of the nuclear cell cycle cascade in experimental mesangial proliferative glomerulonephritis with Roscovitine, a novel cyclin-dependent kinase antagonist. J Clin Invest 1997;100:2512-20. [PubMed]
- Chiara M, Menegatti E, Di Simone D, et al. Mycophenolate mofetil and roscovitine decrease cyclin expression and increase p27(kip1) expression in anti Thy1 mesangial proliferative nephritis. Clin Exp Immunol 2005;139:225-35. [PubMed]
- Li L, Wang H. The cyclin dependent kinase inhibitor (R)-roscovitine prevents alloreactive T cell clonal expansion and protects against acute GvHD. Cell Cycle 2009;8:1794-802. [PubMed]
- Avunduk AM, Varnell ED, Kaufman HE. The effect of roscovitine on herpetic keratitis. Exp Eye Res 2003;76:679-83. [PubMed]
- Milovanceva-Popovska M, Kunter U, Ostendorf T, et al. R-roscovitine (CYC202) alleviates renal cell proliferation in nephritis without aggravating podocyte injury. Kidney Int 2005;67:1362-70. [PubMed]
- Hoogendijk AJ, Roelofs JJ, Duitman J, et al. R-roscovitine reduces lung inflammation induced by lipoteichoic acid and Streptococcus pneumoniae. Mol Med 2012;18:1086-95. [PubMed]
- Aydemir A, Abbasoglu O, Topaloglu S, et al. Protective effect of roscovitine on renal ischemia-reperfusion injury. Transplant Proc 2002;34:2027-8. [PubMed]
- http://meetinglibrary.asco.org/content/33727-65
- Patrick C, Crews L, Desplats P, et al. Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. Am J Pathol 2011;178:1646-61. [PubMed]
- Efficacy Study of Oral Seliciclib to Treat Non-Small Cell Lung Cancer. Available online: http://clinicaltrials.gov/show/NCT00372073
- Zhang R, Liu Y, Zhang J, et al. Intrathecal administration of roscovitine attenuates cancer pain and inhibits the expression of NMDA receptor 2B subunit mRNA. Pharmacol Biochem Behav 2012;102:139-45. [PubMed]
- Yang L, Gu X, Zhang W, et al. Cdk5 inhibitor roscovitine alleviates neuropathic pain in the dorsal root ganglia by downregulating N-methyl-D-aspartate receptor subunit 2A. Neurol Sci 2014;35:1365-71. [PubMed]
- Liu X, Liu Y, Zhang J, et al. Intrathecal administration of roscovitine prevents remifentanil-induced postoperative hyperalgesia and decreases the phosphorylation of N-methyl-D-aspartate receptor and metabotropic glutamate receptor 5 in spinal cord. Brain Res Bull 2014;106:9-16. [PubMed]
- Bukanov NO, Smith LA, Klinger KW, et al. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitorroscovitine. Nature 2006;444:949-52. [PubMed]
- Bukanov NO, Moreno SE, Natoli TA, et al. CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD. Cell Cycle 2012;11:4040-6. [PubMed]
- Zencak D, Schouwey K, Chen D, et al. Retinal degeneration depends on Bmi1 function and reactivation of cell cycle proteins. Proc Natl Acad Sci U S A 2013;110:E593-601. [PubMed]
- Martin KL, Hill GA, Klein RR, et al. Prevention of radiation-induced salivary gland dysfunction utilizing a CDK inhibitor in a mouse model. PLoS One 2012;7:e51363. [PubMed]
- Zoja C, Casiraghi F, Conti S, et al. Cyclin-dependent kinase inhibition limits glomerulonephritis and extends lifespan of mice with systemic lupus. Arthritis Rheum 2007;56:1629-37. [PubMed]
- Kabadi SV, Stoica BA, Hanscom M, et al. CR8, a selective and potent CDK inhibitor, provides neuroprotection in experimental traumatic brain injury. Neurotherapeutics 2012;9:405-21. [PubMed]
- Oumata N, Bettayeb K, Ferandin Y. at al. Roscovitine-derived, dual-specificity inhibitors of cyclin-dependent kinases and casein kinases 1. J Med Chem 2008;51:5229-42. [PubMed]
- Bettayeb K, Sallam H, Ferandin Y, et al. N-&-N, a new class of cell death-inducing kinase inhibitors derived from the purine roscovitine. Mol Cancer Ther 2008;7:2713-24. [PubMed]
- Sroka IM, Heiss EH, Havlicek L, et al. A novel roscovitine derivative potently induces G1-phase arrest in platelet-derived growth factor-BB-activated vascular smooth muscle cells. Mol Pharmacol 2010;77:255-61. [PubMed]
- Houzé S, Hoang NT, Lozach O, et al. Several human cyclin-dependent kinase inhibitors, structurally related to roscovitine, as new anti-malarial agents. Molecules 2014;19:15237-57. [PubMed]