Blocking the ‘MIDAS’ touch of Enterococcus faecalis
Introduction
The treatment and prevention of hospital-acquired infections represents a major challenge to patient care. It is estimated that approximately 1.7 million hospital-acquired infections occur annually in the United States alone, accounting for almost 100,000 deaths and $5-10 billion in associated health care costs (1,2). Catheter-associated urinary tract infections (CAUTI) account for up to 40% of all hospital-acquired infections (3), and thus constitute the greatest burden of acquired disease in the nosocomial environment. The risk of bacteriuria in catheterized patients is estimated to be 5-10% per day (4), and most patients with an indwelling urinary catheter for 30 days or longer develop bacteriuria (5). Multidrug resistance (MDR) is now frequently associated with pathogens that cause CAUTI, leading to increased rates of treatment failure with standard antibiotic therapies and a rise in the use of second and third-line agents. A wide-range of Gram-positive and Gram-negative organisms can cause CAUTI, including Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Staphylococcus spp. and Enterococcus spp. (6). Enterococcus spp., in particular Enterococcus faecalis (E. faecalis), cause up to 25% of all CAUTIs and are increasingly associated with MDR in the nosocomial setting (7-9). Here we review the exciting new findings reported by Flores-Mireles and co-workers, who have defined the mechanism of growth and biofilm formation by E. faecalis in human urine, and translated this new knowledge into the development of a vaccine that can prevent CAUTI in a mouse infection model (10).
The MIDAS motif of Ebp is important for adherence of E. faecalis to fibrinogen
E. faecalis produce a heteropolymeric extracellular hair-like fimbrial structure called the endocarditis- and biofilm-associated pilus (Ebp) (11). Ebp is composed of three subunits; a major repeating subunit that comprises the bulk of the organelle (EbpC), a minor subunit that forms the base of the structure (EbpB) and a tip-located adhesin (EbpA). E. faecalis mutants that do not express the EbpA adhesin, or contain a mutation in the metal ion-dependent adhesion site (MIDAS) motif of EbpA, are attenuated in a mouse CAUTI model (12). Using a series of in vitro biofilm assays, Flores-Mireles et al. showed that the N-terminal domain of EbpA (EbpANTD), where the MIDAS motif is located, is important for mediating biofilm formation, which is dependent on adherence to the extracellular matrix (ECM) protein fibrinogen (10). Fibrinogen-dependent biofilm formation was also ablated following mutation of the EbpA MIDAS motif, demonstrating that this motif is critical for structural conformation of EbpANTD and coordinating E. faecalis adhesion to and colonization of surfaces (Figure 1).
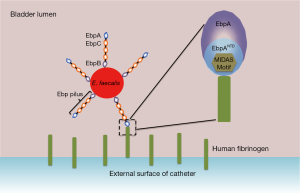
Catheter implantation invokes fibrinogen release in the mouse bladder
What is the source of fibrinogen during infection, and how is this related to catheter implantation? Flores-Mireles et al. investigated the presence of fibrinogen in the lumen of the mouse bladder post-catheterization (10). Accumulation of fibrinogen was detected within 1 hour of catheterization, and increased in a time-dependent manner. By 24 hours, significant accumulation of fibrinogen was observed on the bladder epithelium and on the catheter surface, demonstrating that fibrinogen accumulation represents a major component of the inflammatory response to catheterization. Infection of catheterized mice revealed that wild-type E. faecalis, but not mutants deficient in functional EbpA, adhered to the bladder epithelium and catheter surface. Thus, adherence to remodeled bladder and catheter surfaces coated with host-released fibrinogen via EbpA defines the molecular mechanism of biofilm formation by E. faecalis.
Fibrinogen: more than just a sticky surface
EbpA-mediated adherence of E. faecalis to fibrinogen only partly explains the ability of this pathogen to cause CAUTI. Intriguingly, E. faecalis did not grow in human urine collected from healthy individuals. However, supplementation of human urine with fibrinogen supported growth and the formation of catheter-associated biofilms. In the assays performed by Flores-Mireles et al., an E. faecalis mutant containing a non-functional MIDAS motif in EbpA was unable to form a biofilm in fibrinogen-supplemented human urine (10). Thus, it appears that catheter implantation induces fibrinogen release, and this serves as both an essential growth nutrient and a receptor target for specific adhesion by E. faecalis. More broadly, urinary fibrin/fibrinogen degradation products were reported to be elevated in patients with chronic pyelonephritis (13) as well as during UTI with frank hematuria in children (14). Thus, the role of fibrinogen and its breakdown products in forms of UTI beyond CAUTI may be underappreciated given its complex role in other infectious diseases. Fibrinogen may help to restrain bacterial growth by promoting clearance by host immune cells in some circumstances, but may be detrimental when excessive coagulation promotes bacterial adhesion, survival and dissemination (15). One would also assume that pathogens such as E. faecalis must secrete proteases that can efficiently break down fibrinogen in order to enable its uptake as a nutrient source.
Vaccination with EbpA can prevent CAUTI
The identification of a critical interaction between the EbpANTD and fibrinogen for E. faecalis adherence and biofilm formation suggests that blocking this interaction might represent a novel strategy for the prevention of CAUTI. Flores-Mireles et al. tested this hypothesis by vaccinating mice with EbpA and then challenging these mice with E. faecalis following catheterization (10). The EbpA vaccine was administered to mice intramuscularly with two booster doses at 4-week intervals. This induced a robust and long-lasting response, demonstrating the immunogenicity of EbpA as a vaccine antigen. Vaccinated and control mice were then catheterized and challenged with E. faecalis. Excitingly, this strategy resulted in a 4-log reduction in bacterial burdens associated with both the bladder and catheter in the vaccinated mice. Immunization of mice with other Ebp components—i.e., EbpB and EbpC, or the C-terminal domain of EbpA did not provide protection from catheter-associated infection. Thus, vaccination with EbpA, specifically EbpANTD, can generate a protective immune response by targeting a disease defining EbpA-fibrinogen interaction. This represents a viable alternative strategy for the prevention of CAUTI.
Conclusions and outlook
The increasing incidence of CAUTI caused by multidrug resistant pathogens in the nosocomial setting represents a major challenge to patient health. E. faecalis is one of the most significant causes of device-related infections, and resistance to last-line antibiotics such as vancomycin by this pathogen is of major concern. The study by Flores-Mireles et al. utilized detailed molecular knowledge of a novel host-pathogen interaction, in this case between the EbpA adhesin N-terminal domain and fibrinogen, to develop a new anti-virulence targeted vaccine (10). The work paves the way for more detailed molecular and structural analysis of this key interaction, and the identification of the specific metal ion(s) involved in the activation of the MIDAS motif for providing structural conformation to the EbpANTD. There are important parallels between their approach and other methods currently being developed to prevent UTIs caused by Gram-negative pathogens. Fimbriae-mediated adherence is critical to enable colonization of the urinary tract by all uropathogens. In the case of Gram-negative pathogens such as uropathogenic E. coli, vaccines targeting fimbrial adhesins (16-18), as well as novel therapeutics that block adhesin-receptor interactions (19-21), have been used successfully in animal models (22). These approaches offer a viable alternative to antibiotic treatment. The next step requires the assessment of efficacy in carefully designed clinical trials. If inhibitor molecules could be developed against the EbpANTD of E. faecalis in the same way that mannosides can target the FimH adhesin of uropathogenic E. coli, one could envision a whole new anti-virulence treatment and prevention program for CAUTI. It may also be possible to develop multivalent vaccines composed of a range of adhesins from different uropathogens. Finally, preventing the induction of fibrinogen release, or even other ECM proteins if relevant, may also represent a strategy to limit bacterial growth and adherence in the catheterized urinary tract.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007;122:160-6. [PubMed]
- Stone PW, Hedblom EC, Murphy DM, et al. The economic impact of infection control: making the business case for increased infection control resources. Am J Infect Control 2005;33:542-7. [PubMed]
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control 2004;32:470-85. [PubMed]
- Warren JW, Tenney JH, Hoopes JM, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 1982;146:719-23. [PubMed]
- Stamm WE. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am J Med 1991;91:65S-71S. [PubMed]
- Jacobsen SM, Stickler DJ, Mobley HL, et al. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 2008;21:26-59. [PubMed]
- Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol 2008;29:996-1011. [PubMed]
- Nicolle LE. Catheter-related urinary tract infection. Drugs Aging 2005;22:627-39. [PubMed]
- Ipe DS, Sundac L, Benjamin WH Jr, et al. Asymptomatic bacteriuria: prevalence rates of causal microorganisms, etiology of infection in different patient populations, and recent advances in molecular detection. FEMS Microbiol Lett 2013;346:1-10. [PubMed]
- Flores-Mireles AL, Pinkner JS, Caparon MG, et al. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med 2014;6:254ra127.
- Nallapareddy SR, Singh KV, Sillanpää J, et al. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 2006;116:2799-807. [PubMed]
- Nielsen HV, Guiton PS, Kline KA, et al. The metal ion-dependent adhesion site motif of the Enterococcus faecalis EbpA pilin mediates pilus function in catheter-associated urinary tract infection. MBio 2012;3:e00177-12. [PubMed]
- Tishkov I, Tschoukanov C, Doytshinov D, et al. Fibrin deposits in the glomeruli and fibrin/fibrinogen degradation products (FDP) in the blood and urine in some renal diseases. Int Urol Nephrol 1982;14:89-93. [PubMed]
- Uttley WS, Maxwell H, Cash JD. Fibrin-fibrinogen degradation products in children with renal disease. Arch Dis Child 1974;49:137-42. [PubMed]
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 2012;34:43-62. [PubMed]
- Langermann S, Möllby R, Burlein JE, et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 2000;181:774-8. [PubMed]
- Langermann S, Palaszynski S, Barnhart M, et al. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 1997;276:607-11. [PubMed]
- Roberts JA, Kaack MB, Baskin G, et al. Antibody responses and protection from pyelonephritis following vaccination with purified Escherichia coli PapDG protein. J Urol 2004;171:1682-5. [PubMed]
- Cusumano CK, Pinkner JS, Han Z, et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 2011;3:109ra115.
- Guiton PS, Cusumano CK, Kline KA, et al. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob Agents Chemother 2012;56:4738-45. [PubMed]
- Totsika M, Kostakioti M, Hannan TJ, et al. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J Infect Dis 2013;208:921-8. [PubMed]
- Carey AJ, Tan CK, Ipe DS, et al. Urinary tract infection of mice to model human disease: practicalities, implications and limitations. Crit Rev Microbiol 2015. [Epub ahead of print].


