Mitochondrial dysfunction in Kennedy’s disease: a new pharmacological target?
Introduction
Kennedy’s disease, also known as spinal and bulbar muscular atrophy (SBMA), is a rare inherited lower motor neuron disease characterized by bulbar and proximal limb muscular atrophy. It is caused by a CAG-repeat expansion, encoding a polyglutamine tract, in the first exon of the androgen receptor (AR) gene on the X-chromosome (1). The number of CAG repeat range from 10 to 36 in normal population, while in Kennedy’s disease the expanded repeat ranges from 38 to 62 (2). To date, the abnormal expansion of CAG repeat has been identified to cause nine neurodegenerative diseases including SBMA, Huntington’s disease (HD), dentatorubral-pallidoluysian atrophy (DRPLA) and six forms of spinocerebellar ataxia (3). Although the causative gene varies with each disease, these polyglutamine disorders share common pathways of molecular pathogenesis, such as accumulation of abnormal proteins, transcriptional dysregulation and disruption of axonal transport (3,4). Additionally, several lines of evidence suggest that mitochondrial impairment and elevated oxidative stress are also implicated in the pathogenesis of Kennedy’s disease (5,6). As we know, progression of Kennedy’s disease is slow and the management focuses on preventing complications of the disease. Therefore, targeting mitochondrial dysfunction in Kennedy’s disease may have significant therapeutic potential.
Here, we assessed oxidative stress status in a 51-year-old case who diagnosed with Kennedy’s disease. In particular, we investigated whether the treatment of mitochondrial nutrient (L-carnitine) could improve the disease progression.
Materials and methods
Patients
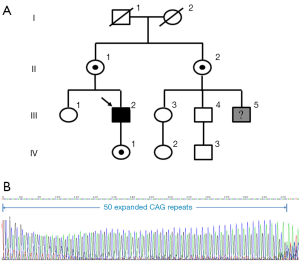
In our study, a 51-year-old man presented to our outpatient clinic with a 10-year history of weakness of proximal lower limbs, as well as with mild swallowing difficulty. His weakness gradually increased and then involved upper limbs about 5-year ago. He also noticed some hand tremor and fasciculation in his bilateral face. His cousin was diagnosed with “Kennedy’s disease” 2-year ago. For genetic analysis, we also recruited eight family members from this pedigree, including three males (III-2; III-4; IV-3) and five females (II-1; II-2; III-3; IV-1; IV-2), across three generations (Figure 1A). All subjects gave informed consent for participation in the investigation. A detailed medical history of the patient was obtained and medical documentation was confirmed. Subsequently, the patient underwent standardized neurologic examination and biologic tests, including serum creatine kinase (CK), cholesterol and hormones level. Electromyography (EMG) was also performed on one distal and one proximal muscle of at least one lower and upper.
Treatment with intravenous L-carnitine (2 g/day) for the patient was started on admission. To assess efficacy, detailed neurologic examination and routine laboratory tests were checked at 2 weeks.
Genetic analysis and plasma 8-hydroxydeoxyguanosine (8-OHdG) measurement
Genomic DNA was acquired from peripheral blood cells of the patient and other family members using conventional techniques. Polymerase chain reaction (PCR) amplification of CAG repeats in exon 1 of the AR gene was performed using a forward primer (5’-TCCAGAATCTGTTCCAGAGCGTGC-3’) and a reverse primer (5’-TGGCCTCGCTCAGGATGTCTTTAAG-3’). The detailed PCR conditions and measurement of the CAG repeat have been previously described elsewhere (1).
Blood samples were collected at our outpatient clinic with fasting. Plasma was separated by centrifugation at 3,000 rpm for 5 min and kept frozen at −80 °C until assayed for 8-OHdG. The plasma 8-OHdG levels of each member was measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Santa Cruz Biotechnology, Inc. Dallas, Texas, USA) according to the manufacturer’s protocol. All samples were assayed in triplicate.
Results
Neurologic examination revealed a mildly nasal quality and tongue atrophy. Proximal muscles of the upper and lower limbs were moderately atrophic. Muscle strength as measured with manual muscle tests of the left upper limb was grade 4+/5, and those of proximal lower limb was grade 4−/5. Sex hormones, thyroid function, rheumatologic antibodies, routine blood and serum biochemistries were normal except for a severe elevation of CK (606.5 U/L; normal value: 15-170 U/L). EMG revealed high amplitude potential, reduced interference and prolonged duration suggesting neurogenic damages.
The CAG repeat number was assessed in the patient as well as eight relatives within the pedigree. Sequencing of the first exon of AR revealed an increased number of CAG repeat (50; the normal range from 10-36) in the case (Figure 1B). We also detected three heterozygous female carriers in this family (II-1; II-2; IV-1) (Figure 1A). Plasma 8-OHdG level in the patient was relatively elevated (34.68±1.01 ng/mL) compared with the female carriers and non-carriers (Figure 2A).
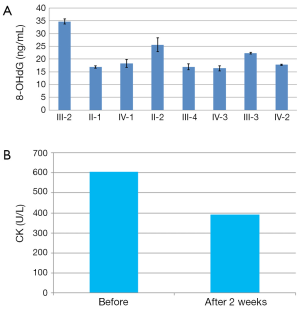
Two weeks after L-carnitine treatment, subjective feeling of muscle weakness was ameliorated to a significant extent. But we observed no prominent recovery of muscle strength of limbs. Follow-up biochemistries test revealed a reduction of approximately 40% in CK level (391 U/L) (Figure 2B).
Discussion
Kennedy’s disease, caused by the expansion of CAG repeat within the AR gene, has been found to be involved in a number of complex and not necessarily independent pathogenesis (4). It is now widely accepted that accumulation of polyglutamine-expanded AR is central to the pathogenesis of Kennedy’s disease. Both the structure of the polyglutamine-expanded AR and its interactions with other proteins are altered relative to the normal AR. The major histopathological hallmark of Kennedy’s disease is loss of lower motor neurons in the spinal cord as well as in the brainstem motor nuclei. Androgen-dependent nuclear accumulation of the pathogenic polyglutamine-expanded AR appears to play a significant role in the neuronal dysfunction and eventual neuronal death (7). Except for nuclear accumulation, polyglutamine-expanded AR is shown to induce the depolarization of the mitochondrial membrane directly though abnormal associations with mitochondria. It also suppresses the transcription of mitochondrial proteins indirectly though alterations in the expression of gene that regulate mitochondrial biogenesis (6). These likely contribute to mitochondrial dysfunction and resulted in increased reactive oxygen species (ROS) generation. 8-OHdG, produced by the reaction between ROS and guanine residues in DNA, is considered to be an indicator of oxidative stress and mitochondrial dysfunction. Increased plasma 8-OHdG level in the case may directly result from the mitochondrial dysfunction-elicited oxidative stress, further raised the awareness of perturbed mitochondrial function in the pathogenesis of Kennedy’s disease. Meanwhile, oxidative stress exasperates the mitochondrial function and restricts ATP production, eventually contributing to neuronal death.
L-carnitine is an important mitochondrial nutrient which acts as a carrier for long chain fatty acids across the mitochondrial membrane. It is necessary for the normal mitochondrial oxidation of fatty acids and subsequent ATP production. Besides, L-carnitine also exerts an effective antioxidant role and may protect tissues against oxidative damage in mitochondrial disorders (8). L-carnitine transports across the blood-brain barrier by an organic cation/carnitine transporter (OCTN2) and accumulates in neurons (9). Thus L-carnitine supplementation may enhance the mitochondrial function of neurons, by the activation of mitochondrial biogenesis and the amelioration of oxidative stress damage. The observed reduction in CK level may present effective treatment of L-carnitine in Kennedy’s disease.
Conclusions
To date, no therapy has proved to be effective on patients with Kennedy’s disease in clinical trials with hormonal therapy showing only limited improvement in certain disease features (10). Consequently, the mitochondrion-specific antioxidant property of L-carnitine possibly represents a new approach for targeting the rescue of mitochondrial dysfunction in the treatment of Kennedy’s disease.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (81471309, 81371406, and 81171209), the Shandong Provincial Outstanding Medical Academic Professional Program, Qingdao Key Health Discipline Development Fund, and Qingdao Outstanding Health Professional Development Fund.
Disclosure: The authors declare no conflict of interest.
References
- La Spada AR, Wilson EM, Lubahn DB, et al. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991;352:77-9. [PubMed]
- Fischbeck KH. Kennedy disease. J Inherit Metab Dis 1997;20:152-8. [PubMed]
- La Spada AR, Taylor JP. Repeat expansion disease: progress and puzzles in disease pathogenesis. Nat Rev Genet 2010;11:247-58. [PubMed]
- Beitel LK, Alvarado C, Mokhtar S, et al. Mechanisms mediating spinal and bulbar muscular atrophy: investigations into polyglutamine-expanded androgen receptor function and dysfunction. Front Neurol 2013;4:53. [PubMed]
- Young JE, Garden GA, Martinez RA, et al. Polyglutamine-expanded androgen receptor truncation fragments activate a Bax-dependent apoptotic cascade mediated by DP5/Hrk. J Neurosci 2009;29:1987-97. [PubMed]
- Ranganathan S, Harmison GG, Meyertholen K, et al. Mitochondrial abnormalities in spinal and bulbar muscular atrophy. Hum Mol Genet 2009;18:27-42. [PubMed]
- Adachi H, Katsuno M, Minamiyama M, et al. Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain 2005;128:659-70. [PubMed]
- Gülçin I. Antioxidant and antiradical activities of L-carnitine. Life Sci 2006;78:803-11. [PubMed]
- Kido Y, Tamai I, Ohnari A, et al. Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-L-carnitine across the blood-brain barrier. J Neurochem 2001;79:959-69. [PubMed]
- Katsuno M, Banno H, Suzuki K, et al. Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:875-84. [PubMed]


