Lung cancer detection with digital chest tomosynthesis: first round results from the SOS observational study
Introduction
Lung cancer is the leading cause of cancer related deaths in most of western and developing countries. Most lung cancers are detected when they are in an advanced stage. Recently, the National Lung Screening Trial (NLST) showed a screening benefit with low dose CT scan (LDCT) in people with a history of heavy smoking, at least for 30 pack years, who are age 55 to 74, current smokers or who have quit smoking within the past 15 years (1). The American Cancer Society, the American Association of Thoracic Surgeons, and the American Society of Clinical Oncology (ASCO) now recommend LDCT screening with a grade of recommendation 2B (2-4). However, according to ASCO, the optimum screening schedule is to be determined (2). NLST demonstrated a reduction in lung cancer mortality of 20% in the LDCT arm compared to the Chest X-ray arm. Screening for lung cancer with LDCT has the potential to detect indolent tumors, resulting in overdiagnosis. The number of cases of overdiagnosis found among the 320 participants who would need to be screened in the NLST to prevent 1 death from lung cancer was 1.38. Whereas the NLST demonstrated a relative mortality reduction with LDCT, the limitations of the screening process, including the magnitude of overdiagnosis, should be considered when guidelines for mass screening programs are constructed (5). However, radiation exposure and cost of LDCT are still a problem. In a previous paper (6), we have shown that chest digital tomosynthesis (DT), may offer an alternative to CT-screening with a detection rate of lung nodules comparable to that reported with LDCT and a lung cancer detection rate of 0.98%, low effective radiation dose to patients (approximately 0.13 mSv) (7) and a cost of approximately 1/6 of that of CT (8). DT uses a conventional radiograph tube, a flat-panel detector, a computer-controlled tube mover, and special reconstruction algorithms to produce section images. Compared with conventional chest radiography, chest tomosynthesis improved sensitivity in the detection of CT-proven lung nodules. DT is able to detect most lung nodules larger than 5 mm, in particular 91% of nodules, whose sizes were between 4 and 6 mm, and 100% of nodules larger than 6 mm, detected in a CT scan. In addition, the effective radiation dosage to patients from chest examination with DT is low (approximately 0.13 mSv compared with 0.1 mSv for a postero-anterior and lateral chest radiograph). Although it lacks the depth resolution of CT, tomosynthesis provides some of the benefits of CT at lower costs and radiation dosages (9). We report the results of the first round results from the Studio OSservazionale (SOS), observational study.
Patients and methods
The study design is reported elsewhere (6), briefly a sample size of 2,000 subjects was planned. Current or former smoker with a smoking history of at least 20 pack years; age 45-75 years; no previous history of cancer in the 5 years before the start of the study; and no chest CT scan study in the 12 months up to enrolment. The mean age was 61 years (95% CI: 48-73); on average, they smoked 580 cigarettes yearly during their life (95% CI: 420-1,400); 29% of the subjects were smokers at the time of accrual. For former smokers, the maximum time since quitting smoking was 10 years. All participants were requested to sign an informed consent form, according to the requirements of the local Internal Review Boards and Health Authorities. Under the study protocol, a chest DT (Volume RAD, GE Healthcare, Chalfont St Giles, England) exam was performed at baseline in all subjects and another 1-year later in those with a negative baseline scan. The images were reconstructed in 3 mm plane spacing in the coronal plane. Image reconstruction was done using the matrix inversion tomosynthesis algorithm (simultaneous algebraic reconstruction technique) and a sliding average of seven adjacent planes was selected to reduce noise and low-contrast tomosynthesis artifacts. The mAs in X-ray were set with an automated control of the exposure to be ten times lower than the ones used for the chest radiograph. A scout image (chest radiograph) was obtained to check patient position; if satisfactory, the system then calculated the appropriate low-dose exposure (mAs) for the tomosynthesis scan. The images fully covered the field from the anterior skin to the back of the chest. As already reported (6), two radiologists independently viewed the tomosynthesis images. One had more than 20 years of experience with chest imaging and 2 years of clinical experience with DT. The other radiologist has 5 years of experience with chest imaging and 1 year of clinical experience with DT. Each nodule was marked on only one image per imaging technique and in the most prominent location. Both observers were instructed to mark the lung nodules on the tomosynthesis images. The observers were allowed to change window width and window level and image contrast and use the pan and zoom functions. All readings were performed on a picture archiving and communications system integrated workstation. The largest diameter in the transverse plane was measured. Subjects with a nodule >5 mm or with multiple nodules followed the same flow chart reported in the previous paper (6). If nodule <5 mm was detected by the DT a 1 year follow-up DT was warranted. An interdisciplinary team including thoracic surgeons, radiologists, and pneumologists reviewed all uncertain cases. All subjects were also followed for any interim diagnosed cancer. Mc Nemar’s test was applied to assess improved observer performance in correctly diagnosed pulmonary nodules. The weighted k statistic was calculated to assess interobserver agreement. Significant differences were defined as P value less than 0.05. Statistical analysis was carried out using Mathematica 8.0 (Wolfram Research).
Results
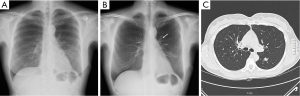
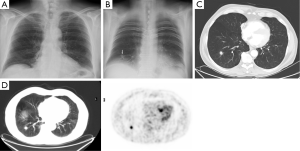
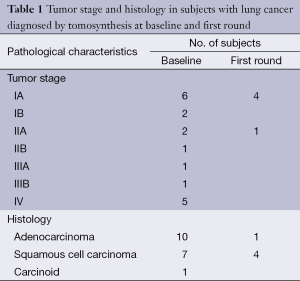
Totally 1,703 candidates underwent first round DT (92% of baseline), the drop out was around 0.4% (68 subjects, most from geographical areas distant from our Center). The mean age was 60 years (95% CI: 49-73). Overall, subjects who had a normal finding at the baseline tomosynthesis examination and those who did not underwent a LDCT follow up underwent a first round DT. Lung nodules >5 mm were detected in 13 (0.7%). The mean diameter of the nodules detected at first round DT were 0.8 mm; the mean diameter was smaller than in the baseline DT (1.7 mm). There were no interobserver differences regarding the sensitivity to detect nodules (P>0.13; Mc Nemar’s test). Only one sub-solid type nodules was detected and was followed with yearly DT. In the baseline round, 14.8% had abnormal findings at DT; the number of nodules found in each patient varied from 1 to 12. In the first round DT, the abnormal findings were 0.7% with number of nodules varied from 1 to 3. None of these 13 subjects had any nodules at baseline DT. At CT scan, one case resulted as false positive (pleural plaque). Twelve subjects underwent further evaluation and a PET/CT study was obtained in 10 subjects (0.6%) while 2 subjects had a follow up schedule (Figure 1). PET scan was positive in all subjects (Figure 2). Surgery, either minimally invasive or thoracotomy for non-diagnosed pulmonary nodules was performed in these 10 subjects. Histological examination of the removed specimen revealed 1 tuberculous nodule, 2 organizing pneumonia, 1 intrapulmonary lymph node increased in size at follow-up CT and 1 lung metastasis from an adenocarcinoma of biliary tree operated more than 10 years before. A lung cancer was diagnosed and resected by VATS lobectomy in 5 subjects (Table 1). There was not any interim diagnosed cancer. The lung cancer detection rate at first round was 0.29% (5/1,703). At the first screen, the prevalence of lung cancer in the SOS study subjects was 23/1,919 (1.20%).
Full table
Discussion
Following the release of the NLST results (1), LDCT has been strongly recommended for the target group of heavy smokers (30 pack-years; 55 to 74 years of age) by a few medical and surgical associations (2-4). However, according to ASCO the optimum screening schedule is to be determined. As previously reported the adsorbed radiation doses and costs associated with LDCT screening encourage the search for other strategies for the early detection of lung cancer or to select among individuals at high risk those who should be referred for LDCT. DT, a computerized evolution of the traditional tomography, or stratigraphy developed by the Italian radiologist Alessandro Vallebona, demonstrated in our previous observational study to be able to detect lung nodules and lung cancer in a risk population in a percentage comparable to the rates reported for LDCT at baseline (0.9%) (6). In the first round, the lung cancer detection rate (0.29%) was lower than other screening trial may be due to the limited number of participants. In our experience, DT allowed to select a subgroup (from 7% at baseline to 0.7% at first round) of patient with lung nodules to undergo CT scan either low dose or contrast enhanced depending from the size of the nodule. DT has been shown to be superior to chest radiography in the detection of pulmonary nodules (7,8). DT was also compared with CT in its ability to detect artificial pulmonary nodules 5 and 8 mm in diameter and Ground Glass Opacities (GGO) in a phantom (10). The detectability indices for tomosynthesis and CT were similar. Although, according to Zhao et al. (11), the GGO 5-8 mm with a density of –800 HU has a detection rate higher with LDCT than with DT. This is, however, a minor issue because GGO <10 mm in diameter have a very long grow rate (12). According to Nakata et al. (13), GGO <10 mm in diameter were in most cases atypical adenomatoid hyperplasia. It has been reported that DT is significantly better than chest radiography at visualizing small nodules in the peripheral lung. In the paper of Izumo et al. (14), DT was conducted in advance and used for mapping the location of the GGO lesion in the coronal plane before bronchoscopy. Real time DT cannot be used for guided bronchoscopy because of the time necessary for image reconstruction. For the improvement of the diagnostic accuracy of DT for GGO, it is useful to develop real-time DT. The reduction of lung cancer specific mortality in a prospective randomized clinical trial was necessary to insure that lung cancer deaths were being prevented by screening (15). In the first round, there was a high rate of surgery for benign lesion. All lesion were PET positive and not existent in the baseline round DT. The slow growing lung cancers detected by screening are almost exclusively non-solid or partial solid nodules that typically correspond pathologically with in situ non-invasive and microinvasive adenocarcinoma with broncho-alveolar features. Detection of new nodules during subsequent annual repeat screens after a baseline screening study is common, in confirmation of the fact that nodules typically grow in the interim between annual screens. Application of well-designed and validated diagnostic algorithms can aid in prevention of overaggressive diagnostic management and overtreatment. Overtesting and overtreatment might also be prevented by modification of the definition of what represents a positive screening result. A comparable increase in size criterion for a cut-point for nodules has been discussed, but is unsupported by published data (16). A further step to ameliorate the DT detection rate of lung nodules and to reduce false positive result will be the Computed Aided Detection (CAD) of lung nodules (15). CAD systems improve the performance of radiologists in the detection process of pulmonary nodules. However, to be used routinely in the radiology department these systems must meet the following requirements: improve the performance of radiologists (high sensitivity in the diagnosis, low number of false positives), have high processing speed, present high level of automation, low cost, and the ability to detect different types and shapes of nodules. Further researches are needed to improve existing systems and propose new solutions. Future CAD systems should improve the level of automation, through integration with picture archiving and communication systems and the electronic record of the patient, decrease the number of false positives, measure the evolution of tumors, evaluate the evolution of the oncological treatment, and its possible prognosis (17). Accurate lung segmentation is important for improving the performance of CAD in chest DT. Wang et al. (16) developed an automated lung segmentation method with good performance that could be useful for the development of CAD schemes.
SOS trial, being an observational study, has no comparison arm. The specificity of DT was very high since 2% were false positive at baseline and only one lesion (0.06%) detected by DT was not confirmed at CT scan in first round. DT examinations led to the discovery other pathological finding in 17/1,919 (0.69%) of subjects: extra-pulmonary tumor, interstitial lung disease, bullous emphysema, pleural effusion, pleural lesion, and mediastinal enlargement due to various reasons (e.g., thoracic aorta aneurism, non-Hodgkin lymphoma). DT could keep a screening program more cost-effective avoiding futile LDCT along with its cost and radiation burden.
Conclusions
The first round results on the use of chest DT in the early detection of lung cancer are as good as for the baseline; the detection rate is comparable to the rates reported for LDCT and is attained at a far lower cost and radiation dose. The first round tomosynthesis screen has provided further data about its effectiveness in the follow-up of high-risk subjects. Large scale randomized controlled trial will be need to confirm DT benefits and identify where it is best used in the clinical setting, and how to further improve the sensitivity and specificity of chest DT. It can be easily understood that, if present data will be confirmed by other studies and clinical trials, chest DT could become the first-line lung cancer screening tool among patients at high-risk of lung cancer (who should undergo a LDCT scan if positive to DT).
Acknowledgements
Study grant-aided by Cassa di Risparmio di Cuneo — Foundation. This study received the FONICAP 2012 Award entitled to Mr. Pasquale Gallo.
The members of the SOS Study Group are as follows: Liliana Comello, MD; Alberto Talenti, MD; Paolo Violino, MD; Grazia Giovinazzo, MD; Claudia Vinay, MD (all Department of Radiology); Andrea Bianchi, MD; Alberto Biggi, MD (both Nuclear Medicine Unit); and Stephan Chauvie (Medical Physics Unit).
The authors thank the medical radiology technicians: Vilma Ceretto; Raffaella Fantini; Cristina Gondolo; Roberta Marchiani; Andrea Oggero; Monica Giubergia; Graziella Enrici; Chiara Maccagno; Roberta Patriti; Chiara Marchesi; Marzia Piola; Maria Stella Sciolli. The authors thank Martina Mormone and Patrizia Massolo for coordinating patients’ recruitment, follow-up procedures, and data management. The authors also thank the “Emilio Bianchi Show” for the cooperation in the recruitment. The institution where the study was performed provided logistic support, telephone lines, software, computer assistance, and an office free of charge.
Disclosure: The authors declare no conflict of interest.
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- American Cancer Society. Guidelines for the Early Detection of Cancer – Lung cancer. Available online: http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. [PubMed]
- Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012;307:2418-29. [PubMed]
- Patz EF Jr, Pinsky P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med 2014;174:269-74. [PubMed]
- Terzi A, Bertolaccini L, Viti A, et al. Lung cancer detection with digital chest tomosynthesis: baseline results from the observational study SOS. J Thorac Oncol 2013;8:685-92. [PubMed]
- James TD, McAdams HP, Song JW, et al. Digital tomosynthesis of the chest for lung nodule detection: interim sensitivity results from an ongoing NIH-sponsored trial. Med Phys 2008;35:2554-7. [PubMed]
- Vikgren J, Zachrisson S, Svalkvist A, et al. Comparison of chest tomosynthesis and chest radiography for detection of pulmonary nodules: human observer study of clinical cases. Radiology 2008;249:1034-41. [PubMed]
- Dobbins JT 3rd, McAdams HP. Chest tomosynthesis: technical principles and clinical update. Eur J Radiol 2009;72:244-51. [PubMed]
- Gomi T, Nakajima M, Fujiwara H, et al. Comparison between chest digital tomosynthesis and CT as a screening method to detect artificial pulmonary nodules: a phantom study. Br J Radiol 2012;85:e622-9. [PubMed]
- Zhao F, Zeng Y, Peng G, et al. Experimental study of detection of nodules showing ground-glass opacity and radiation dose by using anthropomorphic chest phantom: digital tomosynthesis and multidetector CT. J Comput Assist Tomogr 2012;36:523-7. [PubMed]
- Hiramatsu M, Inagaki T, Inagaki T, et al. Pulmonary ground-glass opacity (GGO) lesions-large size and a history of lung cancer are risk factors for growth. J Thorac Oncol 2008;3:1245-50. [PubMed]
- Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT. Chest 2002;121:1464-7. [PubMed]
- Izumo T, Sasada S, Chavez C, et al. The diagnostic utility of endobronchial ultrasonography with a guide sheath and tomosynthesis images for ground glass opacity pulmonary lesions. J Thorac Dis 2013;5:745-50. [PubMed]
- Grannis FW Jr. Minimizing over-diagnosis in lung cancer screening. J Surg Oncol 2013;108:289-93. [PubMed]
- Wang J, Dobbins JT 3rd, Li Q. Automated lung segmentation in digital chest tomosynthesis. Med Phys 2012;39:732-41. [PubMed]
- Firmino M, Morais AH, Mendoça RM, et al. Computer-aided detection system for lung cancer in computed tomography scans: review and future prospects. Biomed Eng Online 2014;13:41. [PubMed]


