
Neoadjuvant immunotherapy for patients with non-small cell lung cancer—current evidence
Shu and colleagues (1) made a significant contribution to a growing body of evidence for the utilization of immunotherapy in the neoadjuvant setting for patients with resectable non-small cell lung cancer (Table 1) (2-4). Of 39 assessed patients, 31 were commenced on treatment consisting of Atezolizumab with nab-paclitaxel and carboplatin. After excluding two patients with colorectal cancer and brain metastasis, 29 underwent surgery with a curative intent. At the time of operation, three patients were deemed unresectable, with 26 patients achieving R0 resection. There was one (3%) mortality from pneumonia and respiratory failure, with major perioperative complications mostly related to neutropenia, liver dysfunction, and thrombocytopenia. Pathological analysis of resected specimens reported that 57% of the intention-to-treat population had major pathological response (MPR), including 33% who had pathological complete response (PCR).
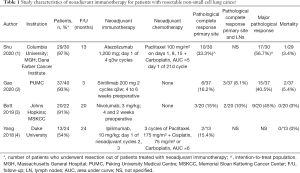
Full table
Compared to previous studies, a significant proportion of patients (10.3%) had exploratory ‘open-and-close’ operations that concluded the tumor was unresectable (2-4). This may reflect a relatively higher proportion of patients (77%) with advanced cIIIA disease, and willingness of the surgeons to attempt challenging resections for larger tumors with mediastinal nodal involvement. Notably, 54% of patients underwent thoracotomy rather than minimally-invasive surgery, reflective of the technical challenges posed by infiltration of inflammatory cells associated with the pseudoprogression phenomenon related to immunotherapy. Previous studies described frequent conversions to open thoracotomies after encountering hostile hilar fibrosis and adhesions (3). In regard to patient selection, non-smokers were excluded from the study, and a relatively higher proportion of patients (40%) with squamous cell carcinoma (SCC) were included, compared to previous studies (3,4). Of the ten patients with SCC who underwent R0 surgical resection, 80% had MPR and 50% had PCR, compared to 53% and 33% of patients who had adenocarcinomas, respectively. This echoed findings by Gao et al., who analyzed neoadjuvant Sintilimab (2). In their report, out of 37 patients who had evaluable pathology, 15 (41%) had MPR, all of whom had SCC, with a strong trend favoring response for SCC compared to adenocarcinomas. Although limited in numbers, these findings support the hypothesis that neoadjuvant immunotherapy may be more effective in patients with malignancies that have a high mutational burden, which may be associated with SCC and smoking history (3,5).
Overall, this study further demonstrated that neoadjuvant immunotherapy was well tolerated prior to surgical resection with an acceptable safety profile. Larger studies in the future should assess the impact of PD-L1 expression and correlation of pathological response with radiological response, which remains uncertain (2,3).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a free submission to the journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5026). Dr. RZ has the following disclosures: Advisor Board – BMS, Pfizer; Speaker Honorariam – BMS, Astra Zeneca, MSD. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 2020;21:786-95. [Crossref] [PubMed]
- Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020;15:816-26. [Crossref] [PubMed]
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Yang CJ, McSherry F, Mayne NR, et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105:924-9. [Crossref] [PubMed]
- Willis C, Fiander M, Tran D, et al. Tumor mutational burden in lung cancer: a systematic literature review. Oncotarget 2019;10:6604-22. [Crossref] [PubMed]


